Features: Manufacturing & Prototyping
In this Q&A, Brian Semcer, president of MICRO, talks to Medical Design Briefs about how the next generation of contract manufacturers is redefining agility, quality, and partnership in the medical device ecosystem. Read now!
News: Green Design & Manufacturing
Cadence, Inc., Staunton, VA, has been awarded a silver medal from EcoVadis, the world’s most trusted provider of business sustainability ratings. This marks the first time Cadence...
Features: Manufacturing & Prototyping
Returning to the Minneapolis Convention Center this October, MD&M Midwest 2025 is where the region’s medtech leaders gather to explore, collaborate, and solve the toughest engineering challenges in medical device development. Read on to learn more about it.
News: Manufacturing & Prototyping
Trelleborg Medical Solutions has opened 43,055 sq ft (4,000 sq m) of manufacturing space in Hal-Far, Malta, helping medical device and life science customers bring...
Features: Manufacturing & Prototyping
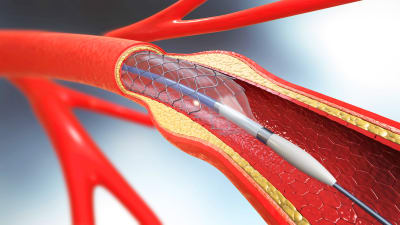
Medical device contract manufacturing is poised for robust and sustained growth, with the global market projected to surpass $150 billion by 2030. This expansion is being driven by technological innovations, shifting healthcare demands, and increasing reliance on outsourcing to improve efficiency and cost-effectiveness. Read on to learn more.
Features: Manufacturing & Prototyping
Contract manufacturing partners play a vital role in the medical device supply chain. Their capability to meet precise design specifications is essential. When they consistently deliver high-quality results on schedule and within budget, they provide substantial value, helping manufacturers reduce risk, accelerate time to market, and maintain regulatory compliance. Read on to learn more.
Briefs: Manufacturing & Prototyping
Through universal models of self-evaluation and data analysis, the LSS methodology has proven to be an excellent tool in fostering major improvements within a business. In the medical device industry, it is particularly important that manufacturers maintain a mindset of continuous improvement and are willing to collaborate in the pursuit of common goals. Read on to learn more about it.
Blog: Manufacturing & Prototyping
As device designs become increasingly sophisticated, medtech companies are understandably seeking contract design and manufacturing partners that can accompany them on...
Applications: Medical
Boston Scientific is a market leader in pacemakers, defibrillators, monitoring equipment, spinal and brain stimulation, stents, catheters, and ablation devices. Read on to learn how the company improved upon cardiac monitoring.
INSIDER: Medical
Intricon Corporation, developer and manufacturer of some of the world’s smallest, smartest medical devices powered by microelectronics, has announced a significant expansion at its St....
Features: Manufacturing & Prototyping
The expertise of CDMs that have dedicated space for innovation are focused on accelerated development cycles, DfM, and the optimal application of materials science for their OEM partners. Read on to learn more.
News: Design
Cadence, Inc., a provider of vertically integrated contract manufacturing solutions to the medtech and pharma markets, is excited to announce a significant milestone in the company’s...
News: Medical
Vantedge Medical, a portfolio company of Aterian Investment Partners, has acquired Hobson & Motzer, Inc. Founded in 1912 and based in Connecticut, Hobson is a manufacturer...
Features: Test & Measurement
While mechanical testing may play a small role in the total scope of CDMO competencies, a poorly equipped solution can result in significant issues with time management, data integrity, and root cause analysis. Investing in highly capable testing systems should be considered a long-term investment and serve to showcase a commitment to quality for potential clients. Read on to learn more.
News: Manufacturing & Prototyping
Flambeau Medical, a full-service contract manufacturing organization serving the medical device and life sciences industries, has added a new industrial 3D printer to expand its rapid...
Products: Manufacturing & Prototyping
See the products in the Product Showcase, including Omnetics’ Nano-D connectors, Cadence's complex medical devices, Formacoat's medical device coating application, the Lee Company’s Full Isolation Dual Seal Precision Dispense Pump, Ulbrich’s Braid Wire Accelerator® Program, and much more.
Features: Medical
The global economy is changing, and for the first time in more than half a century, economic reality is bringing manufacturing back to the United States. Global inflation, coupled with vulnerabilities in the supply chain exposed by the pandemic, makes manufacturing in the United States cheaper and less risky.
News: Manufacturing & Prototyping
Trelleborg Medical Solutions has launched an Innovation Center in Plymouth, MN. An expansion of the company's former Rapid Development Center, it is designed to...
News: Manufacturing & Prototyping
Teleflex Incorporated, a global provider of medical technologies, has announced the completion of its 8,200-square-foot extrusion expansion at the Teleflex Medical OEM facility located in...
News: Medical
Vander-Bend Manufacturing, Inc. and its subsidiaries including TMK Manufacturing , JL Haley, Swiss Precision Machining, and Omni Components have merged to become one...
Blog: Medical
Understanding what the FDA really means by their time frames, knowing how to address their regulatory issues, and preparing a response to a 483 or Warning Letter is critical to showing the FDA that you understand what they expect from you and that you will work to bring your company into compliance. Another critical point is to understand that you can negotiate with the agency.
News: Manufacturing & Prototyping
Flambeau Medical Markets Group, part of the Nordic Group of Companies, has partnered with Two Styx Capital (TSC). The company will continue to operate as Flambeau Medical...
News: Materials
Cadence, Inc., a provider of design, development, and contract manufacturing services to the medical device, drug-delivery, and diagnostics markets, has acquired the Florida location of...
Features: Medical
The experience of technology providers in developing minimally invasive surgical instruments and RAS systems assembly offers significant advantages for the leading manufacturers of robot-assisted surgical systems.
Briefs: Medical
Additive fusion technology opens doors to more sustainable manufacturing options that can help lower cost and reduce weight and waste, all while maintaining the integrity and strength required of geometrically complex and small parts subjected to harsh environments.
Briefs: Medical
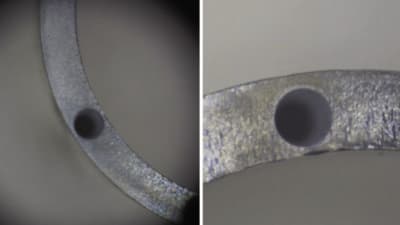
As the medical device manufacturing industry continues to innovate in the form of improved tolerance requirements and an increased usage of advanced materials, manufacturers should consider the merits of a nonconventional material removal process like PECM.
Podcasts: Design
Medtech companies must adapt to a landscape that is now data-centric systems vs. equipment-centric.
News: Manufacturing & Prototyping
Sterling Medical Devices and RBC Medical Innovations has rebranded the combined company as Vantage MedTech. According to the company, leveraging decades of industry...
News: Manufacturing & Prototyping
Critical Manufacturing, a developer of manufacturing execution systems (MES) for global, multi-site operations, has expanded into Mexico, reinforcing its commitment to delivering...

 Podcasts: Medical
Podcasts: Medical Podcasts: Design
Podcasts: Design Podcasts: Design
Podcasts: Design On-Demand Webinars: Medical
On-Demand Webinars: Medical Podcasts: AR/AI
Podcasts: AR/AI Podcasts: Manufacturing & Prototyping
Podcasts: Manufacturing & Prototyping