As device designs become increasingly sophisticated, medtech companies are understandably seeking contract design and manufacturing partners that can accompany them on comprehensive product journeys — from initial concept to validation to large-scale manufacturing, and everything in between. This places a differentiating premium on partners with the ability to combine ideation and early-stage development with materials science and process technologies.
This relatively recent dynamic runs decidedly against the historical grain. In a globalized supply chain that frequently relies upon numerous design and production partners providing separate components for complex, mission-critical medical devices, a single-partner approach seems almost pollyannish. However, the added value offered by truly turnkey partners — ones that can steer a complicated, multi-component medical device from materials selection and prototype engineering straight through ramp-up production and broader commercialization, with participation in critical steps along the way — has never been more apparent.

This value is amplified for medical device categories comprising a “subset within a subset.” As the overall healthcare solutions landscape becomes more intricate, more medtTech companies are forgoing singularly focused specialists for multilane partners featuring cross-discipline, even concept-to-commercialization capabilities. This “under one roof” mindset favors contract development and manufacturing organizations (CDMOs) with holistic strategic skillsets rather than piecemeal tactics that must be stacked upon elsewhere.
If too many chefs can ruin an entrée, too many design, engineering, and production partners can limit and delay the realization of a complex medical device. Nowhere is this consolidation-centric concept truer than with advanced stents and catheter products.
Stents & Catheters: A Hyper-customized Pairing
A comprehensive dissertation encompassing the myriad varieties of stents and catheter combinations would require a book rather than a few pages, so instead let’s explore one sliver of this market — a “subset within a subset within a subset,” so to speak. Let’s take a deep dive into a category known for its heightened levels of design, engineering and manufacturing challenges: neurovascular stent systems.
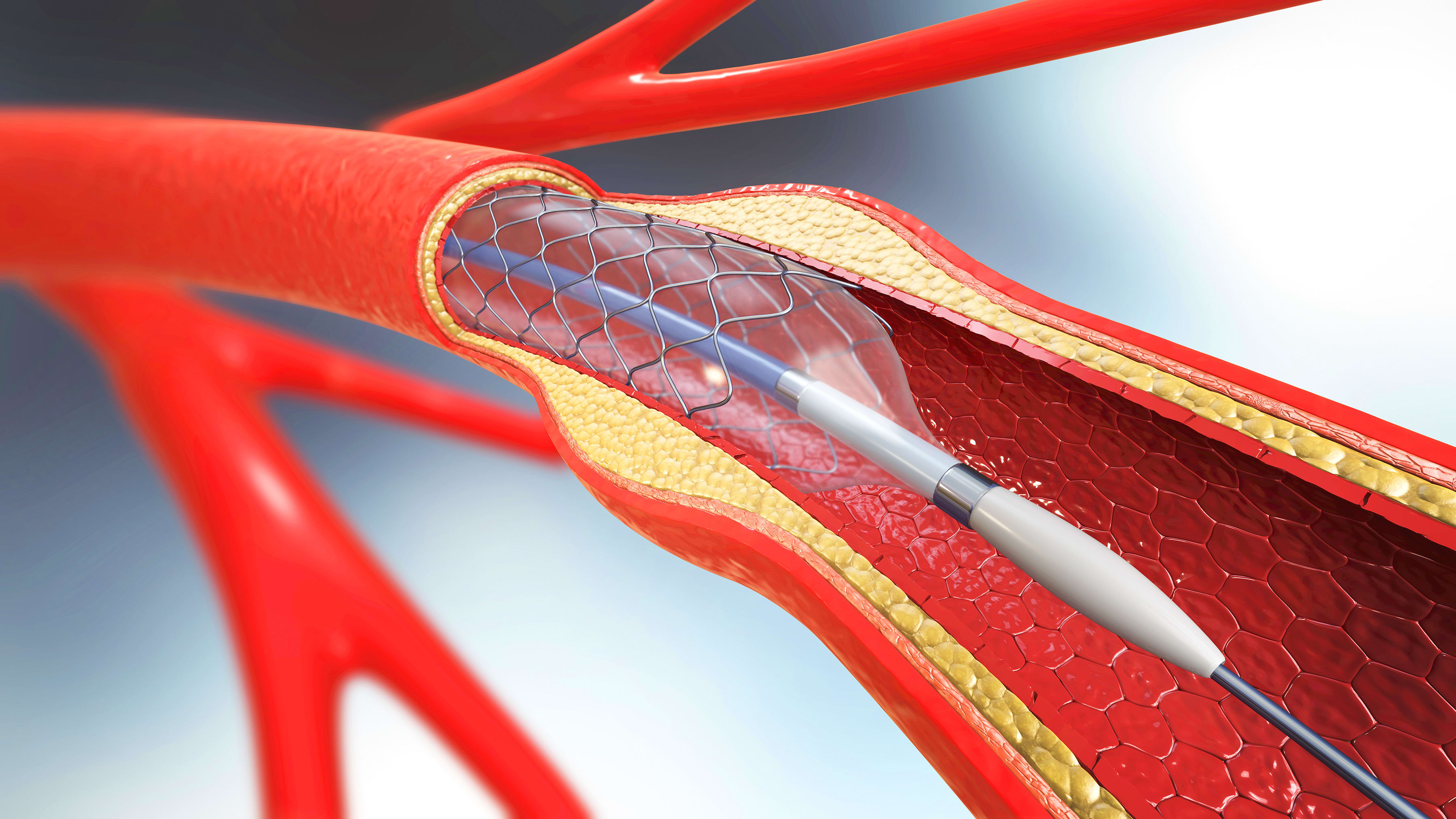
All stents are minuscule; neurovascular stents are exceedingly so, frequently measuring just two or three millimeters. The tiny tubular devices are implanted within blood vessels in the intracranial cavity to treat a vascular abnormality, such as those resulting from aneurysms or strokes.
Here, the bird’s-eye view becomes the proper perspective. Neurovascular stents are an exceptionally intricate device that, after being coupled with another device (a catheter), becomes the focal point of a delicate, difficult medical procedure. The entire process — not only design through manufacturing but design through implantation — must be as optimized as possible. This optimization requires not just individually perfected steps but the synchronization of those steps.
Whereas many stents come preloaded into their complementary catheter delivery systems, neurovascular stents occupy too limited a landscape for such conveniences. Given the narrow, twisting tortuous path that must be navigated for successful placement, preloading a neurovascular stent in a catheter would make the latter too stiff for the task at hand.
Rather, neurovascular stents are placed by first inserting a small-diameter microcatheter, typically about 150 cm in length. Once the microcatheter reaches its destination, the guidewire is removed; the stent is then fed through the distal end and, ever so gently, pushed to the precise implant spot.
Obviously, this process places a premium on the interaction between a neurovascular stent and its microcatheter. The tracking must be smooth and free of bunching, and the final step — the removal of the stent from the microcatheter’s tip — must be seamless.
Already, we can see the pitfalls of a multi-partner building block approach. The various parties involved would need constant, ultradetailed inter-organizational communication to ensure best possible compatibility between the stent and its delivery device. In this example, optimized stent concepting and prototyping must consider more than the attributes of the stent itself. While stent-specific elements like size, radial force and crimping characteristics are invaluable, equally invaluable is understanding how that stent will conform to its delivery vessel: the catheter.
For applications as intricate as stents, catheters must be designed with their payload in mind. The right catheter will meet its stent’s needs for column strength without an overabundance of compliance, because if it stretches too much or too unpredictably, the procedure becomes more complicated and less informed. Deployment accuracy also is critical; for example, a particularly springy stent will need a custom designed catheter mechanism to prevent premature deployment.

With catheters and stents, there are typically several design trade-offs when holistically considering the tortuous path, stent radial force, and catheter compliance. Striking the right balance among these attributes will yield an optimized stent-catheter combo whose procedural application is repeatable, and whose ultimate effects are positive and enduring.
Notably, such intricacies apply not only to stent-catheter combos but also a wide array of adjacent and next-generation solutions. For example, it’s becoming increasingly common for complex catheter systems to include stent-like devices like stentrievers and other devices for thrombectomy. Unsurprisingly, such sophisticated constructions also benefit greatly from extensive experience in traditional stents, catheters, and other interventional medical devices.
Finally, it’s worth noting that catheter design also must consider compatibility with other devices besides stents, including interactions with ancillary, non-proprietary components such as guidewires, introducers and guide catheters.
Lead Actors: Setting the Stage for Successful Implantation
All this leads to one destination: the operating room. While the stent may be the star, the stage is just as important. Considering the complexities of stent-catheter combinations — and neurovascular stent implantation in particular — healthcare personnel conducting such procedures take on crucial supporting roles. Everything from stent design to catheter compatibility to skillful implantation must coalesce in support of one goal: successful acute deployment and long-term device viability.
Can this process be successfully conducted through a multi-partner approach? Certainly. But there are indisputable insights and best practices that can be more thoroughly developed and honed with an overarching view of how both stents and catheters are designed, produced and utilized. The whole of the process is greater than the sum of its parts.
One substantial benefit to containing the entirety of the development and prototyping process in one facility is expedient trial and error. Savvy designers often have in-house simulation labs that precisely mimic how doctors would utilize the stent-catheter combo. This can inform potential modifications to stents, catheters or both.
Compatibility simulations and the foresight they afford are but one area in which medical device CDMOs can showcase value to potential medtech customers. Further upstream from such prototype trials, CDMOs with in-house materials testing and analysis programs help medtech companies understand parameters and potential pitfalls before component construction even commences. Here, experience matters; an outsourcing partner with firm roots in stent-catheter development can draw upon a deep well of knowledge and seasoned team of engineers who’ve successfully designed, produced and launched unique yet similar combinations.
Indeed, even in scenarios involving novel, patented designs, ingrained niche knowledge helps a stent-catheter development process hit the ground running for enhanced speed to market. For example, new stent and catheters concepts typically require approval from regulatory authorities. This includes design stress tests, such as how a stent will handle the strain of crimping. Here, expertise becomes an expediting agent, because even new designs have similarities with existing ones. This means that certain device modeling parameters can be largely informed from an established knowledge base of stent development.
The human body is an exceptionally complicated, interconnected design. Companies providing invasive or implanted medical devices naturally must develop their products in relation to this — and, crucially — according to the operatory procedures used to introduce or employ them. With so many considerations to juggle, more medtech companies are finding reassurances with turnkey partners, whose comprehensive product and process expertise make them less likely to drop the ball.
This article was written by Andrew Filachek, Vice President of Engineering for TekniPlex Healthcare .



