
Contract design and manufacturing organizations (CDMOs) play an increasingly crucial role in the pharmaceutical supply chain, providing the necessary capabilities and capacity to meet growing patient demand. The recent emergence of GLP-1 class drugs only emphasizes the importance of CDMOs, which con- tribute significant expertise related to fill-finish operations, secondary packaging, and distribution.
Understanding their unique requirements is critical to providing CDMOs with mechanical testing systems capable of keeping up with their intensifying pace of business. It is crucial to develop technologies and solutions that allow CDMOs to remain agile to customer requirements without sacrificing the reliability necessary for a production environment.

Across the industry, there are certain features that pharmaceutical clients look for when partnering with a CDMO. Three of the primary features include the manufacturer’s capabilities, agility, and speed of problem resolution. Although these features are important to all aspects of the CDMO’s business, examining them through the lens of mechanical testing allows us to understand how CDMOs can create a more holistic approach to meeting vendor needs in preparation for becoming a pharmaceutical partner. Throughout this article, the functional testing of autoinjectors will be used for GLP-1 class drugs as an example.
Capability
At first glance, the simplest requirement of a CDMO is that it has the capabilities to evaluate the key essential performance requirements (EPRs) of a client’s product. From a client’s perspective, the answer is a simple yes or no. However, from a CDMO’s perspective, their answer may be slightly more nuanced. In many cases, the key question is not just whether the CDMO can meet a client’s requirements, but how it can meet those requirements.
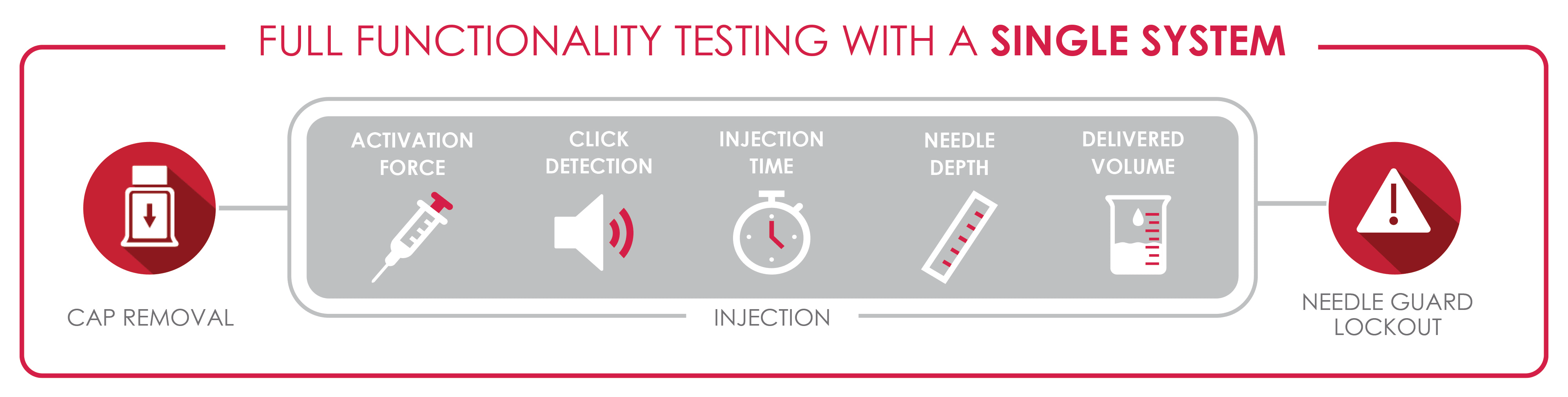
The EPRs for autoinjectors typically include assessing the full range of cap removal force, activation force, injection time, needle depth, delivered volume, patient feedback, and passive safety mechanisms. To adequately evaluate these met- rics, many different measurement devices are required, including a load cell, balance, machine vision camera, and a microphone. A basic approach to performing this testing would be to use multiple testing systems, each configured with a different measurement device.
However, in practice, this introduces many challenges for a CDMO. Firstly, considering these devices are commonly single use, multiple device specimens must be tested to collect all the required data. Not only is this costly and wasteful, but it also leads to the second issue, which is that data must be aggregated across all the separate systems to create a single comprehensive report. The time required for testing and analysis is also significant, considering that a batch of 60 or 100 devices may need to be fully evaluated at a given time. There are also the financial burdens associated with maintaining and operating multiple systems and performing their annual calibrations, in addition to the cost of the device specimens themselves. In this case, the CDMO can technically meet their client’s needs, but not in an efficient or cost-effective way.

The ideal implementation of an autoinjector testing pro- gram utilizes automation and intentional design to test each of these parameters in a sequential run of a single device. Integration of multiple measurements devices onto a single mechanical testing system allows for a streamlined testing process, capable of evaluating the full suite of EPRs in approximately one minute depending on the length of the injection itself. The use of a single system also ensures the data collection is all encompassing and can produce a single batch report for review. Ultimately, this advanced level of capability shows a potential client a commitment to data integrity, testing efficiency, cost awareness, and a thorough understanding of testing requirements.
Agility and Flexibility
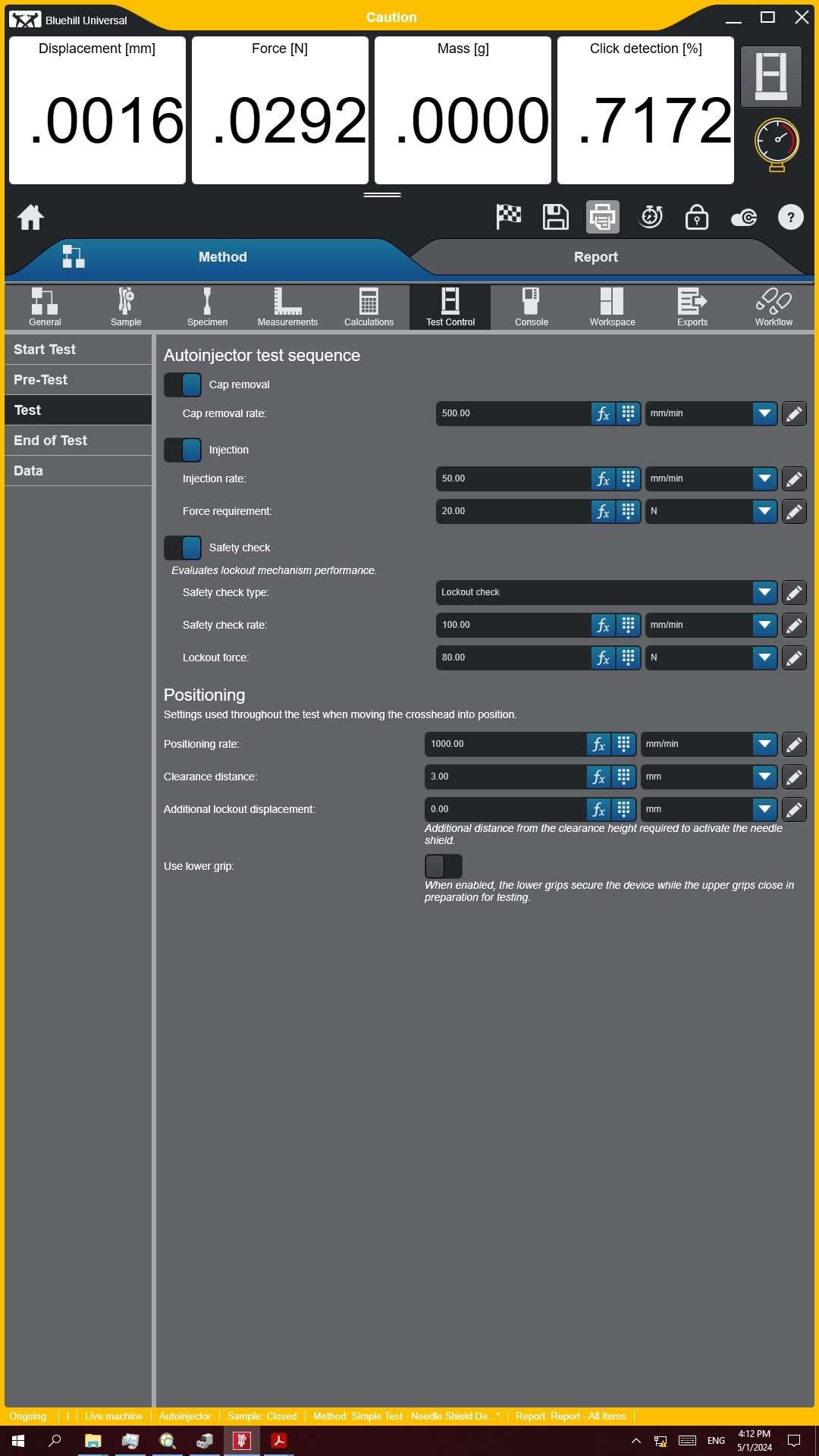
During the ramp up phase, when bringing a program to production readiness, a significant amount of communication takes place between client and their CDMO. This communication includes modifications to testing parameters, acceptance criteria, and, in rare cases, device specifications. It is imperative that the CDMO be able to implement these changes quickly and accurately with minimal vendor involvement. In terms of mechanical testing systems, these modifications will most often manifest in the system method (or recipe as it is sometimes called).
Especially when considering autoinjector testing, there are many different parameters which may be modified, so investing in a test system with an intuitive and customizable machine interface will be important. The machine interface should also be simplistic in its design, ensuring the user can change what they need but isn’t at risk of accidentally changing other parameters. For full flexibility and agility, the CDMO must have full ownership of their test methods. They should be capable and empowered to make the necessary modifications independently of vendor support. The following interface shows an example of setting up a functional autoinjector test, with only the necessary callouts for critical test parameters in industry standard terminology. The ease of method setup and modification ensures CDMOs can confidently address dynamically changing requirements.
Speed of Problem Resolution
In any production environment, device failure can mean significant delays in the batch release and affect the client’s confidence in the CDMO. Any device failure will be treated as mission critical and result in an extensive root failure cause analysis. Depending on the data available, this analysis could take weeks or even months to complete. As such, CDMOs need to invest in technologies which provide visibility into failures, and integrated tools to identify issues after they occur. The quicker the analysis can be performed, or an exception be identified, the shorter the resulting delay in release and shipment.
Intelligent Testing Capabilities
The more EPRs a device includes the greater opportunity there is for something to go wrong in the data collection. Autoinjectors are inherently complex devices with many different modes of failure. Because failure can come from the powerpack, fill volume, primary container, or one of many other components, simply reviewing the data produced by the system is in most situations not sufficient to understand how or why something was out of specification. Qualitative assessments are required to appropriately identify the root cause.
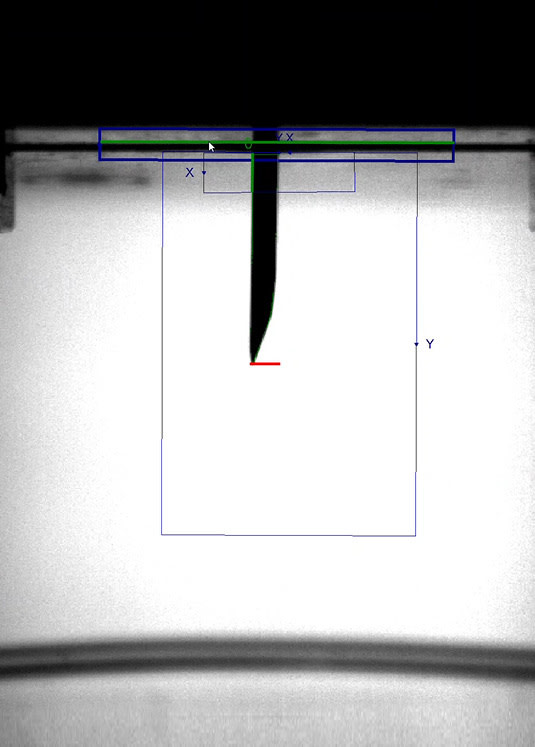
Autoinjector testing systems should be equipped with visual aids to capture the injection event and allow quality engineers to review the information. Injections are not uniform processes and have many different variables that can affect the measurement of EPRs. Video footage can be utilized to capture unexpected failures such as a needle protruding at an excessive angle causing the fluid to miss the collection beaker and result in abnormally low delivered volume. Needle depth can be incorrectly measured if there is a lingering drop of fluid on the needle tip. Capturing images and video during testing, to be used at the time of analysis, can support quickly finding these root causes.
The software should also offer built-in safeguards to ensure all modifications are tracked in a traceable audit log and with the necessary security to delineate who in the organization can make the changes. Especially in the beginning of a program, as operators are being onboarded, the security permissions can ensure that the methods are revision controlled and that the operator will only have access to the most recent version. Providing the program and quality managers with the necessary software tools to oversee and audit their method development will ensure all client requests are addressed and implemented.
Conclusion
While mechanical testing may play a small role in the total scope of CDMO competencies, a poorly equipped solution can result in significant issues with time management, data integrity, and root cause analysis. Investing in highly capable testing systems should be considered a long-term investment and serve to showcase a commitment to quality for potential clients. Quality teams should emphasize the importance of systems which enable efficient root cause analysis, and project managers should actively seek systems built around intuitive user interfaces to maximize flexibility. The decision-making process around mechanical testing systems should demand a comprehensive assessment of their larger impact on meeting client needs and reflecting the drive for organizational success.
This article was written by Landon Goldfarb, Biomedical Marketing Manager at Instron, Norwood. MA. For more information, visit here .
Transcript
00:00:05 Developed in close partnership with pharmaceutical device manufacturers, Instron’s latest generation autoinjector testing system can perform full functionality testing on a wide range of drug delivery devices, including needle shield and button activated devices, as well as safety syringes. Testing can include dose accuracy, activation force, injection time, needle depth, and needle guard lockout. Allowing labs to meet internal quality requirements and international standards such as ISO 11608. Due to the variety and complexity of these different tests, labs often require the use of multiple pieces of test equipment. This approach requires more equipment, more time, and creates a need for data consolidation
00:00:50 with Instron’s next generation autoinjector testing system, labs can run a complete sequence of tests on a single system. Saving money on equipment, maintenance, and specimens. Most importantly, it reduces testing time and simplifies tech transfer. Accelerating time to market. At the start of testing, the system uses the auto-positioning feature to check that the crosshead is in the proper starting position, as defined by your test method. After loading your device into a cap insert and confirming in Bluehill Universal, pneumatic grips close to secure the device and initiate the test sequence. The system initially measures cap removal force
00:01:27 before a pneumatic shuttle moves the cap out of the test space prior to injection. then the head moves downward to engage the needle shield, and a microphone captures the audible click indicating activation of the device. A machine vision camera is used to monitor the needle during the test, capturing needle depth and injection time in Bluehill Software. Using this camera to measure injection time enables the system to be used for higher viscosity materials, such as biologics. This optical solution is better suited to analyze longer or intermittent injection profiles over more traditional laser based or gravimetric systems. Images are captured at the start and completion of injection,
00:02:09 providing a visual tool to confirm the points at which measurements were taken And video of the test is also captured, Allowing playback of the injection to perform root cause analysis in the case of device failure. the system includes a precision balance to measure the fluid mass in real time, determining delivered volume. It can also provide injection time using a custom developed algorithm. to ensure an accurate dose measurement. A burst of air is used to blow off any liquid that remains on the needle tip. The final sequence is a safety check on the device's lockout mechanism. The crosshead moves up, locking out the needle shield before moving back down to either determine the defeat force
00:02:50 or simply ensure the lockout mechanism is functioning properly. The base unit features a swing door for quick access to the collection beaker, and a special base ensures the beaker is properly aligned and prevents it from slipping out from under the injection site. As a safety measure, a light curtain is used to protect operators if the beam is broken at any point during testing, all frame movement stops and the system reverts to a safe setup mode to accommodate a range of devices with minimal changeover. The universal cap Removal base uses interchangeable inserts that fit the most common device geometries, with the option to customize as needed.
00:03:28 The user friendly Bluehill software has a dedicated test method type for autoinjectors. Whether creating a new method or modifying an existing method for a new device, simply input the proper dimensions for the device and adjust the test parameters as necessary. Measurements, including load, mass, and noise detection can be viewed in real time throughout the test. Bluehill’s System Suitability feature enables users to perform required daily verification checks on the load cell, machine vision, camera and scale. The system prompts the operator to complete these checks based on requirements set in the administration settings.
00:04:05 A report of the system suitability results is stored as a PDF, and the successful completion of these checks is recorded in the audit log of the traceability module, Offering an efficient way to comply with the audit requirements of FDA 21 CFR part 11 and other accrediting bodies. When it comes to validation of your system, Instron service can provide onsite verification as well as IQ/OQ validation packages to accelerate the in-house process. The Instron autoinjector testing system simplifies the testing process and empowers users confidently test a wide range of devices. Giving labs more control over their test methods and data analysis tools to generate
00:04:46 better insights into their products and accelerate their testing programs, all serving to improve device quality and ultimately, the patient experience Instron - The difference is measurable.



