News: Regulations/Standards
Medical Design Briefs and MedAccred Team Up to Support Medical Manufacturing Excellence at MD&M West 2026
Features: Manufacturing & Prototyping
Inside the OEM: Medtronic’s Growth and Next Wave of Innovation
Products: Materials
New Products and Services
Briefs: Regulations/Standards
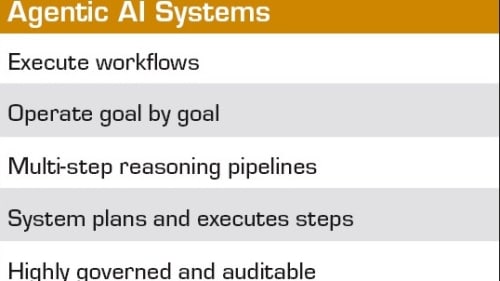
FDA Deploys Agentic AI to Modernize Regulatory Workflows
From the Editor: AR/AI
From the Editor: FDA’s Big AI Moment Signals a New Era for Medtech
Features: Robotics, Automation & Control
MD&M West Show Preview
Features: Electronics & Computers
Engineering for the Future: The Trends Transforming Medtech in 2026
Top Stories
Ask the Expert
John Chandler on Achieving Quality Motion Control

FAULHABER MICROMO brings together the highest quality motion technologies and value-added services, together with global engineering, sourcing, and manufacturing, to deliver top quality micro motion solutions. With 34 years’ experience, John Chandler injects a key engineering perspective into all new projects and enjoys working closely with OEM customers to bring exciting new technologies to market.
Webcasts
 Podcasts: Medical
Podcasts: Medical
How Wearables Are Enhancing Smart Drug Delivery
 Podcasts: Design
Podcasts: Design
Developing Sustainable Drug-Delivery Devices
 Podcasts: Design
Podcasts: Design
Smarter Pathways for Precision Drug Delivery in Cancer Care
 On-Demand Webinars: Medical
On-Demand Webinars: Medical
Understanding Testing and Compliance Requirements for Wireless Medical Devices
 Podcasts: AR/AI
Podcasts: AR/AI
Personalized Medicine and Drug Delivery