
From the herbal remedies of ancient times to the groundbreaking therapies that reshaped medicine in the 20th century, the story of pharmaceutical innovation has been nothing short of remarkable. But one challenge has plagued the industry: The time it takes to get treatments from lab to patient.
Imagine the impact if lifesaving drugs could reach patients in months rather than years. More lives saved, more effective treatments delivered and more business success in the process. Today, that vision is becoming reality. By building a fully connected and digitalized value chain where data flows seamlessly to support faster, smarter decisions, pharma leaders can accelerate development, lower costs and deliver critical therapies in months instead of years. This article explores the essential ingredients of pharmaceutical success, from research and design to development, production and ultimately, the patient.
Digital Threads Connect the Data
Precision is paramount in the pharmaceutical industry, which is why progress has traditionally been cautious and slow. But in today’s market, prolonged timelines and profitability no longer align. Every day a drug launch is delayed can cost companies up to $1.5 million.
So how can companies overcome this barrier? By combining the real and digital worlds and becoming a digital enterprise. This approach enables pharmaceutical organizations to accelerate development without sacrificing quality. Digitalizing their entire product and production life cycles creates a continuous flow of data across design, realization and optimization, enabling faster decisions, greater flexibility and efficiency and improved outcomes.
The digital enterprise empowers companies to unlock the full potential of their data, turning it into actionable insights for smarter, faster and more sustainable operations. But before intelligent decisions can be made, organizations need a strategy: which data to prioritize, where to begin and how to maximize insights. The answer lies in digital threads.
Like a subway map showing the fastest routes, digital threads guide businesses through digitalization by connecting data, processes and people to ensure every step is efficient and effective. They streamline knowledge and technology transfer from lab to pilot to industrial scale and ultimately to commercial manufacturing, allowing scientists to experiment freely while systems capture and organize data.
In pharma and life sciences, digital threads create a continuous flow of information that accelerates design, development, manufacturing and equipment engineering, helping companies win the race against time.
Optimized Design and Development
In pharma and life science, moving quickly can save lives, but speed cannot come at the cost of precision. Traditional development relies on physical experiments that are costly, time-intensive and prone to setbacks. Digital simulation offers a different path.
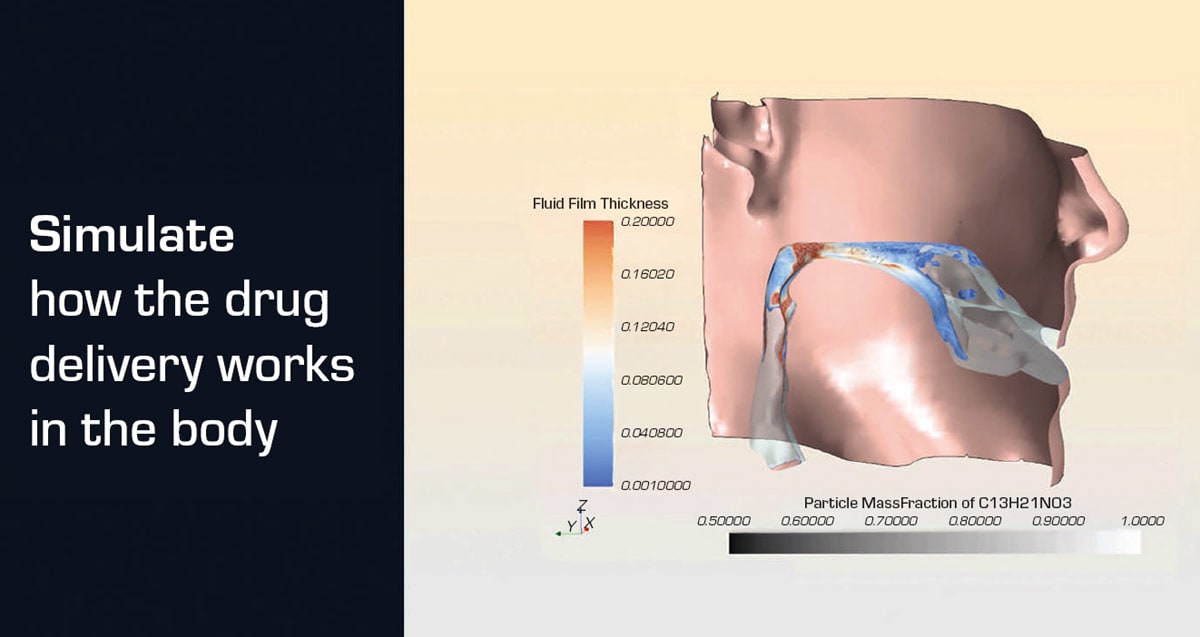
With the comprehensive digital twin, drug design and development can be accelerated without sacrificing quality. Scientists can model medicines at the molecular level and test how different formulations might behave in the body before ever entering a lab. Variables such as particle size or tablet composition can be evaluated virtually, helping ensure that each treatment performs as intended.
Simulation lets researchers test drug formulations and explore the best ways to deliver therapies to the body, without the high cost of physical trials. This simulation helps pharma companies quickly decide whether a drug should be an inhaler, pill or capsule, making it easier to serve smaller, targeted patient groups with life-saving treatments.
Now, with artificial intelligence (AI), companies can leverage AI-driven drug development. With a digital twin, pharma and life science companies can simulate drug production at both lab and industrial scale. This lets manufacturers fine-tune key parameters like volume, temperature and mixing speed without relying on costly physical trials.
By using graphics processing units (GPUs) instead of traditional CPUs, researchers can run hundreds of simulations per hour, cutting development time. AI can even learn from past results to generate insights and streamline future experiments, eliminating the need to start from scratch each time.
Pharma companies utilizing these approaches are already seeing real results such as:
30 percent less raw material used.
50 percent faster time to market.
70 percent lower energy use thanks to GPUs.
Together, these advances shorten R&D timelines and speed the path from lab to patient.
Accelerated Pharmaceutical Manufacturing
Tech transfer from lab to production can take up to three years, but that timeline can be dramatically shortened by repurposing R&D data. With smarter knowledge transfer, life science companies reduce the number of physical experiments needed and move into production faster. This approach allows for quicker scale-up of drug recipes across facilities, improving efficiency at every step.
A digital enterprise makes this possible by ensuring data flows consistently across the entire lifecycle. When companies harness their data effectively, they gain true industrial intelligence.
In pharma and life science, production is divided into two phases. The first phase, primary manufacturing, focuses on producing active pharmaceutical ingredients. This stage is critical, and with the power of digital tools, it can be both accelerated and refined.
Data-driven insights help engineers design and optimize industrial-scale processes by refining factors like material properties, temperature, and pressure. Even the plant layout can be tailored around these optimized parameters. A digital twin then simulates plant operations, allowing teams to test and improve performance virtually, before any equipment is installed. As production begins, the digital twin continues to support real-time adjustments and continuous optimization.
Digitalization also improves the administrative side of pharma. Traditionally burdened by paper-based processes, the industry can now shift to paperless manufacturing. Replacing physical records with electronic batch records increases transparency, boosts quality and supports sustainability.
Add AI to the mix, and efficiency increases. Generative AI tools can draft documentation, ensure regulatory compliance and create clear electronic work instructions — all in a fraction of the time. In an industry where there is a skilled labor shortage and speed, accuracy and compliance are non-negotiables, automating repetitive tasks allows teams to focus on what matters most. Increased flexibility with adaptive production
What if the demand for a certain life-saving drug increases without warning? How can manufacturers scale quickly and meet this demand without risking quality or stalling operations? Adaptive production is the solution. Adaptive production allows pharma companies to respond to market changes fast. Using a modular, plug-and-play strategy enabled by Modular Type Package (MTP) technology, they can add prevalidated, preconfigured process units into existing systems. This makes it easy to adjust formulations, scale batch sizes or switch products without requiring major process changes.
By connecting physical systems with their digital ones, adaptive production expedites automation deployment and enables smooth scaling. Organizations that have adopted this approach have seen results such as:
Up to 50 percent faster time-to-market.
Up to 80 less engineering effort.
Up to 80 more manufacturing flexibility.
Accelerated equipment engineering.
With active pharmaceutical ingredients developed, the focus shifts to the second phase of production, secondary manufacturing. This stage is where drugs are transformed into finished products like syringes, capsules, or tablets. Filling and packaging also occur here. To ensure speed, precision, and quality, companies rely on smart, digitally enabled machinery — and machine builders are key to making that happen.
Using the comprehensive digital twin, machine builders can design and optimize equipment to meet the industry’s high demands. Here’s how:
Faster commissioning: Machines are virtually designed, simulated, and validated before they’re physically built, ensuring a first time-right approach.
Faster training: digital twins simulate real scenarios to help train, reskill and upskill operators — even before the machine arrives.
AI-powered engineering: Industrial copilots generate and optimize complex code, reducing workload, and speeding up development.
Smarter energy use: IT/OT integration reveals energy consumption patterns, helping reduce waste and improve efficiency.
These benefits are only possible with the digital twin and industrial AI. Organizations that are already leveraging these solutions have seen undeniable benefits including:
Up to 50 percent faster workforce training.
Up to 70 percent faster machine commissioning.
Up to 40 percent lower energy consumption.
These solutions enable companies to save weeks, months, or even years, ultimately accelerating the delivery of life-saving medications to patients who can’t afford to wait.
The Future of Pharma and Life Science
Thanks to advances in data, AI, and the digital twin, the pharmaceutical industry is evolving — faster development, smarter production, and tailored therapies are now within reach. These technologies are helping pharma companies deliver innovative, safer treatments to smaller, more targeted patient groups — including personalized medicine.
Pharma is moving beyond one-sizefits-all. With additive manufacturing (like 3D printing), companies can develop highly personalized treatments that adapt to individual needs. For example, if a patient can’t swallow pills, 3D printed formulations can offer alternative delivery — like predissolving the drug in the mouth. These innovations also allow scientists to control drug release more precisely, improving therapeutic outcomes.
The next frontier for pharma is the Industrial Metaverse — a lifelike representation of the entire manufacturing plant with the comprehensive digital twin, industrial AI, and data, as well as software-defined automation. It offers a real-time view of operations, allowing pharma teams to test, optimize, and collaborate virtually, no matter where they are. With one unified platform, companies can spot issues early, simulate changes before implementing them, and drive innovation across the value chain.
Enterprise Recipe Management
Bringing all data together into a single, connected view gives pharma and life sciences companies the speed and clarity to close the gap between R&D and production — shortening the path from lab to patient.
An Enterprise Recipe Management (ERM) approach, powered by end-to-end digitalization, enables seamless, knowledge-based transfer of recipes from development to manufacturing. By automating repetitive tasks, improving consistency, and freeing teams to focus on high-value work, ERM delivers more than faster time to market: it builds the agility needed to adapt to change.
ERM delivers measurable impact across the entire product and production lifecycle:
Faster innovation and drug development.
Streamlined manufacturing.
Stronger quality control.
Better collaboration across teams.
Scalable production, faster.
Pharma’s Race Against Time Is a Winnable One
Digital transformation is giving pharmaceutical and life sciences companies new ways to accelerate discovery and delivery. Tools like AI-driven simulation, adaptive production, and ERM make it possible to bring therapies to market more quickly, align them to individual needs, and scale them responsibly. Companies that adopt these approaches are positioning themselves to lead the next chapter of patient-centered innovation.
This article was written by Tsvetelina Nikolova, Digital Enterprise Marketing Manager, Siemens Digital Industries, Plano TX. For more information, visit here .



