
As healthcare delivery shifts toward decentralized and patient-managed models, medical device engineers face increasing pressure to design compact, connected, and highly reliable drug-delivery systems. Electronic drug-delivery pens are central to this evolution, empowering patients to self-administer life-sustaining therapies safely and consistently outside of hospital settings.
Developing these devices, however, requires navigating multiple engineering challenges. Designers must ensure accurate monitoring of dosing events, extend battery life with ultra-low power electronics and minimize overall size to maximize patient comfort and portability. At the same time, the integration of secure wireless connectivity has become essential for real-time data sharing with caregivers and healthcare providers. Achieving this balance demands not only innovative component technologies, but also careful system-level integration to keep devices efficient, robust, and compliant with stringent medical standards.
This article highlights how emerging component solutions — optimized for low power, small footprints, and wireless performance — are helping design teams meet these challenges. It also examines strategies for leveraging supplier expertise, application support, and compliance resources to accelerate development and ensure long-term reliability in next-generation drug-delivery platforms.
Electronic Drug-Delivery Pen Overview
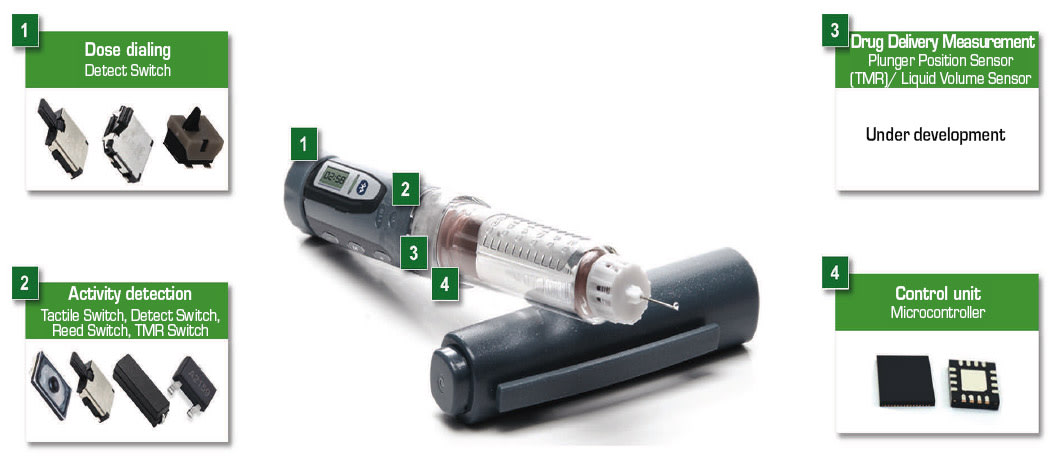
Figure 1 illustrates an example drug-delivery pen. Blocks surrounding the pen indicate the components that provide efficient control functions. Figure 2 presents a block diagram of the electronic drug-delivery pen. The table on the right of the block diagram lists control components recommended for use in the circuit blocks.
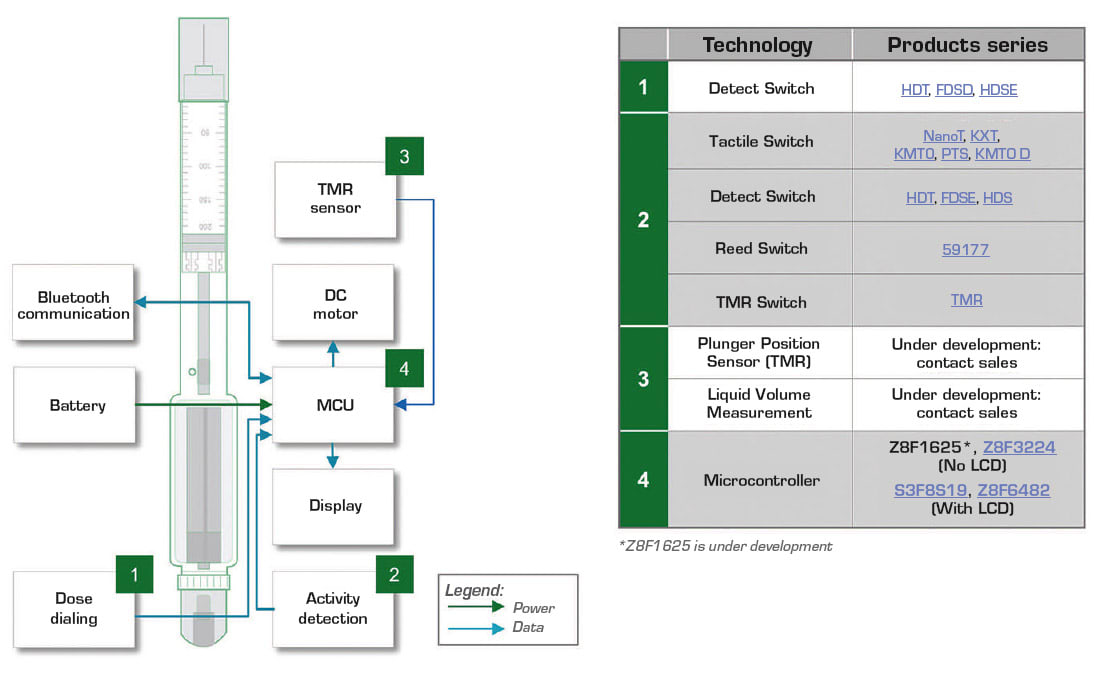
A battery powers the drug-delivery pen’s circuitry. The battery can be a lithium-ion coin cell for minimal space consumption. A compact switch permits detection of the dosage dial position. Additional switches ensure the cap is removed to expose the needle, the needle is in contact with the user’s skin and the correct quantity of medication is delivered.
The microcontroller (MCU) receives inputs from the switches and drives the actuator which injects a drug dose. The actuator can be a small DC motor or a spring mechanism. A spring actuator reduces cost and complexity compared with a motor drive. The MCU also transmits data to the display and interfaces with the Bluetooth circuit to transmit injection information and receive setting adjustments from remote medical personnel.
Efficient, Compact Control
Minimizing power consumption and space requires efficient, small sensing, and control circuit blocks. The options described in the following paragraphs will assist the designer in achieving these two design goals.
Dose Dialing Circuit. The dose dialing circuit requires a switch to indicate the position of the dose dial. Surface-mount detect switches that are as small as 3.5 mm wide × 2.8 mm deep × 3.35 mm high and have a low activation force of 35 g are available. These switches have a low momentary contact resistance of 500 mΩ and a life exceeding 100,000 cycles. Designers can select top or side activation.
Activity Detection Circuit. The activity detection circuit performs several functions, including safety indications of the presence of the drug vial and verification that the injector output is in contact with the patient’s skin. This circuit also activates drug injection and performs drug dose monitoring. Designers have options for detecting these conditions.
One option for sensing is a small, low-profile, top-actuated, surface-mount pushbutton switch. Its dimensions are 2.5 mm wide × 1.65 mm deep × 0.55 mm high. Sealed switches are rated IP67 against an ingress of moisture and dust. Pushbutton switch life cycles can be as high as 300,000 cycles and contact resistance is low at 500 mΩ. A second option is the type of detect switch suggested for the dose dialing circuit.
A third option for the various sensing functions is a reed switch. Designers can specify ultra-miniature, hermetically sealed reed switches with variable sensitivities. Magnetic actuation does not draw any power from the circuit, and the insulation resistance is 1012 Ω, which holds leakage current to pA levels.

A fourth option is a tunneling magnetoresistance (TMR) switch. It consists of a magnetic tunneling junction sensor and CMOS circuits. This magnetically triggered digital switch combines high sensitivity and ultra-low power consumption. Figure 3 shows a block diagram of a TMR switch. A temperature-compensated regulator circuit ensures voltage stability for the internal circuits. A Schmitt trigger provides switching hysteresis for noise rejection, and a CMOS push-pull circuit provides the output drive. The TMR switch is placed next to the bottom of the injector’s plunger chamber. A small magnet mounts on the injector’s plunger. As the actuator pushes the plunger toward the bottom of the injector and the TMR switch, the switch produces an increasing output voltage and switches logic state. The switch indicates the plunger’s position. The MCU uses position information to determine the injected drug volume.
The TMR switch draws only 200 nA when powered by 1.8–5 V circuits. Its sensitivity can detect a magnetic field as low as 5 Gauss. The CMOS output enables direct connection to an MCU, which avoids the need for additional components. The TMR switch is available in very small SOT23-3 or LGA-4 packages. Its high sensitivity, compact size, and low battery drain make it a superior solution for drug dose monitoring. Also, continuous glucose monitors (CGMs) that interact with a drug-delivery pen can use TMR switches to support an optimum therapy protocol.
TMR Sensor. Two technologies are under development for direct measurement of injected liquid volume. One technology employs a new TMR sensor for plunger position measurement. A second technology uses a capacitive sensor and an electrode on the pen that is not in contact with the cartridge. The electrode has a unique patent pending design. TMR and capacitive sensing can provide a more reliable and accurate measurement of injection volume.
Microcontroller. Control of the drug injection pen requires a microprocessor or a microcontroller. Single-chip MCUs can provide all the necessary functionality including monitoring plunger position or directly measuring delivered liquid volume, draw low power and save space. MCU sleep modes can significantly lower power consumption during periods of inactivity.
High-efficiency 8-bit MCUs are available for controlling pen operation. When all peripherals are inactive, available MCUs can have an operating current as low as 5 mA. Lower clock speed settings can reduce the operating current even further. These MCUs have low-power inactivity modes that reduce current consumption to 0.7 μA.
Models can have 10–15 analog-digital converter input channels with 10-, 12-, or 14-bit resolution, 16–64 Kbyte flash memory, and an on-chip temperature sensor array. The MCUs have high-resolution timers for the PWM drive of motors. They also have peripheral I/O ports optimized for interfacing with wireless communication chipsets. Models can also have LCD drivers to control a display. All this built-in capability can be housed in enclosures as small as a 32-QFN (5 × 5 mm) package.
A few versatile, small-sized and high-performance components can enable designers to create the electronics for a fully functional drug-delivery pen. These components allow the circuitry to squeeze into a small package convenient for users.
Standards for Drug-Delivery Pens
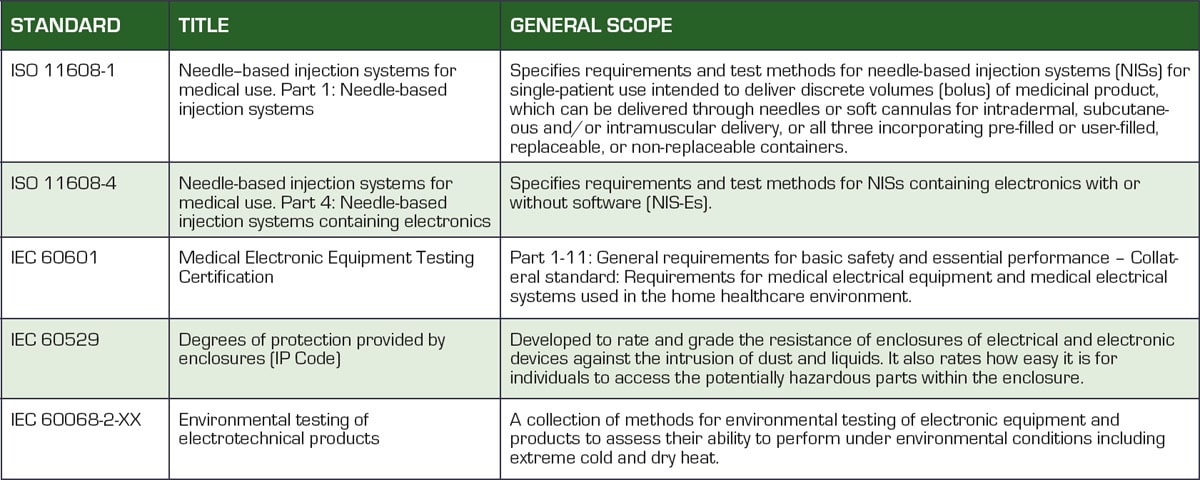
Medical devices are highly regulated to ensure patient safety. Table 1 lists the global standards with which design engineers must comply to develop a certified device. Understanding these standards and their impact on device design is essential for obtaining medical device certification from the regulating bodies.
Resources for Efficient Design
The design of electronic drug-delivery pens requires precise control, compact form factors, and exceptionally low power consumption — requirements that can only be met by thoughtful component selection and close collaboration with experienced suppliers. Surface-mount, energy-efficient components now make it possible to achieve these targets while maintaining compliance with strict regulatory standards.
In addition to advanced components, engineering support services play a pivotal role. Application engineers can help select cost-effective solutions, provide simulation and modeling to optimize device performance, and guide compliance strategies to streamline testing. Precompliance evaluations offered by manufacturers can prevent costly redesigns and shorten time to market. For medical device developers, pairing innovative components with expert resources creates a proven pathway to designing compact, reliable, and patient-friendly drug-delivery systems that support the expanding role of self-care in modern healthcare.
This article was written by Dr. Marco Doms, Sr. Manager Technical Marketing, Littelfuse, Inc., Chicago, IL. In his current role, Doms is responsible for several platforms with entirely new products or product features that require additional internal and customer coordination. For more information, visit here .



