Features: Test & Measurement
Not only are medical devices expected to function as intended, they must meet ergonomic, safety, FDA and functional requirements. They must be designed to function in adverse...
Features: Manufacturing & Prototyping
Medical device manufacturers operate in a challenging environment filled with stringent regulatory requirements and industry pressures. With a rise in mainstream competitors...
News: Regulations/Standards
Health Canada, the Canadian medical device market regulator, is set to require all reprocessed single-use medical devices to Canadian Medical Device Regulations (CMDR) by September 1, 2015. According to MEDEC, the national association created for the Canadian medical technology...
INSIDER: Regulations/Standards
The FDA recognizes that the progression to digital health offers the potential for better, more efficient patient care and improved health outcomes, which requires that many medical devices be interoperable with other types of medical devices and with various types of health information technology...
Features: Regulations/Standards
Medical device manufacturers frequently face unique industry challenges, including the need to manage quality processes across disparate sites or...
Features: Electronics & Computers
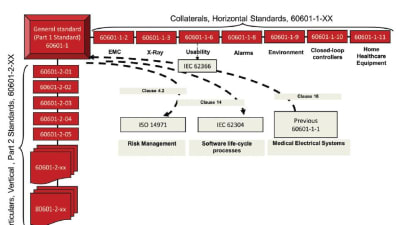
The third edition of IEC 60601-1 has been in effect since June 2012. It replaces the previous version as a basic standard for medical electrical equipment and describes the general...
Technology Leaders: Medical
While a number of countries have standards in regards to overall medical equipment, a few countries have related component requirements (e.g. plugs...
Products: Manufacturing & Prototyping
Compliance West, San Diego, CA, which manufactures custom hipot and surge testers, introduces the D5-P medical tester, specifically designed to conduct the energy reduction test in IEC 60601 Third Edition...
News: Medical
A final guidance, “Design Considerations for Devices Intended for Home Use,” issued by the FDA, is intended to assist manufacturers in designing and developing home-use devices that comply with applicable standards of safety and effectiveness and other regulatory requirements.
News: Regulations/Standards
Scientists at the National Institute of Standards and Technology, Gaithersburg, MD, in collaboration with the National Cancer Institute and the Radiological Society of North America, have designed and developed image-calibration technology to study the effects of traumatic brain injury (TBI). The...
News: Medical
To strengthen the safety of medical devices, the FDA has finalized recommendations to manufacturers for managing cybersecurity risks to better protect patient health and information. On October 1, the agency issued a final guidance on the content of premarket submissions for the management of...
Features: Regulations/Standards
Many medical devices, both existing and new designs, are laser-based. For some time, lasers have been designed into medical devices from diagnostic,...
News: Regulations/Standards
On August 13, The FDA issued a guidance for industry and FDA staff called “Unique Device Identification System: Small Entity Compliance Guide”. This guidance is intended primarily to provide information to the medical device industry, including small businesses, concerning FDA’s September...
News: Medical
The U.S. Food and Drug Administration has issued new draft guidance that would exempt certain Class I and Class II medical devices from premarket, or 510(k),submission requirements. If a device is “exempted from premarket notification,” then the FDA would allow a device that was...
Features: Regulations/Standards
To design and successfully sell medical devices, manufacturers must ask themselves a number of key questions. What range of functions should this product offer? In what type of...
News: Electronics & Computers
UL (Underwriters Laboratories), Northbrook, IL, announced that the FDA has recognized two UL battery safety standards as consensus standards for medical devices incorporating lithium or nickel-based batteries. The two standards are UL 2054 - Standard for Household and Commercial...
News: Medical
Recognizing that the need for effective cybersecurity to ensure medical device functionality has become more important with the increasing use of wireless, Internet- and network-connected devices, and the frequent electronic exchange of medical device-related health information, the FDA has issued a draft...
News: Medical
The U.S. Food & Drug Administration (FDA) has issued the first phase of its Global Unique Device Identification Database (GUDID): Guidance for Industry on June 11, 2014. To quickly provide industry with information critical to successful use of the GUDID, the FDA is issuing the GUDID Guidance in two...
News: Medical
The FDA has recently issued a draft guidance titled, "Humanitarian Device Exemption (HDE): Questions and Answers." This draft guidance answers commonly asked questions about Humanitarian Use Devices (HUDs) and the Humanitarian Device Exemption (HDE) authorized under section 520(m) of the...
Features: Design
Medical devices manufactured today require significant attention to safety and human factors engineering that has not always been exercised in medical device design. IEC safety...
Technology Leaders: Electronics & Computers
Every day, medical device manufacturers are getting better and better at managing risk. They know they have to. Changes have been introduced into international regulatory schemes that impact device design all...
INSIDER: Medical
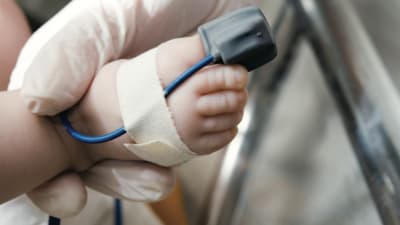
Medical devices are not always one size fits all, especially with regard to child-sized devices. The Philadelphia Regional Pediatric Medical Device Consortium (PPDC) brings together engineers and biomedical researchers from the Children’s Hospital of Philadelphia (CHOP), Drexel...
News: Medical
ASTM International, formerly known as the American Society for Testing and Materials (ASTM), West Conshohocken, PA, has proposed a new international standard to be used in the design, development, quality control, and comparison of a variety of collagen-based medical products, including surgical implants, delivery...
News: Medical
ASTM International, formerly known as the American Society for Testing and Materials (ASTM), West Conshohocken, PA, has proposed a new international standard to be used in the design, development, quality control, and comparison of a variety of collagen-based medical products, including surgical...
News: Medical
The American National Standards Institute (ANSI) has launched the ANSI IBR Portal, an online tool for free, read-only access to voluntary consensus standards that have been incorporated by reference (IBR) into federal laws and regulations.
News: Medical
All interested parties are welcome to join in the development of a proposed new ASTM International standard that will provide guidance on how to conduct axial, bending, and torsional fatigue testing of stents. The proposed new standard, ASTM WK23330, Guide for in vitro Axial, Bending and...
News: Regulations/Standards
Today, the FDA issued its final rule on the unique device identification (UDI) system that, once implemented, will provide a consistent way to identify medical devices. The UDI system should improve the quality of information in medical device adverse events reports, which will help the FDA identify product problems more...
News: Regulations/Standards
The FDA Center for Devices and Radiological Health, Office of Science and Engineering Laboratories, Center for Biologics Evaluation and Research issued a Guidance document on “Radio Frequency Wireless Technology in Medical Devices” containing recommendations to assist industry and...
Features: Electronics & Computers
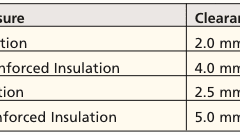
Insulating and jacketing material options for wire and cable are innumerable, even if the field is narrowed to those with some qualification for use in medical electronics....

 Podcasts: Medical
Podcasts: Medical Podcasts: Design
Podcasts: Design Podcasts: Design
Podcasts: Design On-Demand Webinars: Medical
On-Demand Webinars: Medical Podcasts: AR/AI
Podcasts: AR/AI Podcasts: Manufacturing & Prototyping
Podcasts: Manufacturing & Prototyping