Features: Test & Measurement
Manufacturers producing medical devices that involve patient contact are typically required to perform biological safety evaluations, including biocompatibility tests...
From the Editor: Regulations/Standards
By the time you read this, Robert M. Califf, MD, a recognized global leader in cardiology, clinical research, and medical economics, will have been installed as the U.S. Food and Drug Administration’s...
INSIDER: Medical
The International Organization for Standardization (ISO), Geneva, Switzerland, late last month released its long-awaited revision to ISO 13485, the global standard for medical device quality management...
INSIDER: Regulations/Standards
The FDA is seeking comments from the medical device industry and healthcare community that refurbish, recondition, rebuild, remarket, remanufacture, service, and repair medical devices.
INSIDER: Regulations/Standards
Dr. Robert M. Califf, a cardiologist, researcher, and founder of the Duke Clinical Research Institute, was confirmed by the Senate on February 24 as the next Commissioner of the U.S. Food and Administration...
Features: Medical
Some of the biggest stumbling blocks encountered by medical device firms on the way to clearance or approval of their devices by the U.S. Food and Drug...
Features: Regulations/Standards
When it comes to medical equipment, nothing is more important than the safety of patients and health care personnel. From diagnostic tools such as ultrasound devices to home health...
From the Editor: Regulations/Standards
According to the late, great David Bowie, “the stars look very different today”. After two years of collecting the 2.3% Medical Device Excise Tax, the tax has now been suspended for all of 2016 and 2017 when President Obama signed the Consolidated Appropriations Act of 2016. The tax was expected to raise almost $30 billion over...
INSIDER: Connectivity
Cybersecurity threats to medical devices are a growing concern that present a potential risk to the safety and effectiveness of medical devices. To address this, the FDA has issued...
INSIDER: Medical
In a draft guidance for industry and staff issued on December 31, the FDA proposed notifying the public about medical device “emerging signals that the agency is monitoring or analyzing,...
INSIDER: Regulations/Standards
The FDA’s Center for Devices and Radiological Health (CDRH) announced the 2015 Experiential Learning Program (ELP) General Training Program, which is intended to educate CDRH staff regarding the policies, laboratory practices, and challenges faced in broader disciplines that impact the medical...
INSIDER: Medical
IEEE, Piscataway, NJ, has announced a new standard and two new standards development projects designed to support plug-and-play, interoperable communications across eHealth devices. The new eHealth standard is IEEE 2410™-2015, Biometrics Open Protocol Standard,...
From the Editor: Regulations/Standards
Crowdfunding is being used every day to raise money for various causes and to aid in the launch of new products. Spurred on by themes such as “Be the change you want to see in the world,” donors feel virtuous for helping to fund a child’s medical bills or helping a non-profit to provide clean...
INSIDER: Regulations/Standards
On October 14, the FDA issued a draft guidance document to assist industry in designing evaluation strategies for, and reporting the results of, animal studies for medical devices. The studies utilized for the assessment of these devices typically provide initial evidence of device...
From the Editor: Regulations/Standards
Recently, an online discussion board broached the subject of FDA approval for a diagnostic app that might be able to predict when a patient’s condition would relapse. The question postulated whether there was a way to circumvent FDA approval by launching the app in another country and making it available online. And, if...
INSIDER: Regulations/Standards
While historically, the development of new technologies to improve patients’ lives has relied upon experts’ opinions rather than asking patients and families directly what they consider important, this may be about to change. Patients and their care partners are becoming intimately...
INSIDER: Medical
OpenFDA is releasing a treasure trove of information on medical devices that could help spur innovation and advance scientific research. OpenFDA’s Application Programming Interface (API) expands the previous resources about medical device-related adverse events and recalls by incorporating information...
Briefs: Regulations/Standards
In September 2013, the FDA announced new regulations for medical device manufacturers known as UDI (Unique Device Identifier) that would require all medical devices to bear a label...
Briefs: Packaging & Sterilization
Ensuring that device packaging meets specifications.
Considering the complex science and research that goes into developing medical devices, it is important...
INSIDER: Medical
The FDA is seeking companies to take part in a pilot program that would allow medical device manufacturers to report malfunctions of certain low- and medium-risk devices on a quarterly basis. This pilot program will help the agency develop criteria for quarterly malfunction reporting...
INSIDER: Medical
The Emergo Group recently posted an advisory that the FDA has revised the fee charged for issuing medical device export certificates in response to higher costs and demand for these documents.
Technology Leaders: Regulations/Standards
Collaboration among healthcare technology stakeholders—from device manufacturers and healthcare delivery organizations to healthcare security intelligence...
From the Editor: Regulations/Standards
In mid-July, the House of Representatives passed HR 6, also known as the 21st Century Cures Act, which expedites research and development on debilitating diseases and makes it easier to get important treatments to the patients who need them. Among other things, it makes research collaborations easier, reforms and streamlines...
Briefs: Regulations/Standards
The FDA is seeking comments of a new draft guidance that it recently issued for industry called “Adaptive Designs for Medical Device Clinical Studies.” An “adaptive design” for a medical device clinical study is defined as a clinical trial design that allows for prospectively planned modifications...
INSIDER: Medical
The U.S. Food and Drug Administration has withdrawn 47 draft guidance documents published before December 31, 2013, which were never finalized or acted on. The announcement was posted in the Federal Register, stating that this move was made to improve the efficiency and transparency of the guidance...
Technology Leaders: Connectivity
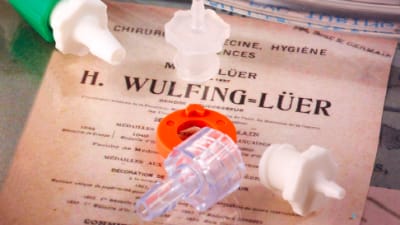
After a 20-year effort to establish standards which would minimize the risk of medical misconnections, the pending release of the ISO 80369 series of standards has now...
Briefs: Medical
The FDA recently adopted three nanotechnology standards as part of a major update to the administration’s List of Recognized Standards. The documents comprise a Technical Specification (TS) developed...
Features: Regulations/Standards
Particulate testing of cardiovascular medical devices is an important and valuable step...
INSIDER: Medical
Medical device regulators at the FDA have issued correcting amendments to their post-market electronic Medical Device Reporting (eMDR) requirements to eliminate any disparities between those rules and their Unique Device Identification (UDI) system.

 Podcasts: Medical
Podcasts: Medical Podcasts: Design
Podcasts: Design Podcasts: Design
Podcasts: Design On-Demand Webinars: Medical
On-Demand Webinars: Medical Podcasts: AR/AI
Podcasts: AR/AI Podcasts: Manufacturing & Prototyping
Podcasts: Manufacturing & Prototyping