Blog: Medical
The U.S. Food and Drug Administration (FDA) took action May 25, 2020, to make more ventilators available to healthcare personnel in the United States. FDA issued an emergency use...
Blog: Regulations/Standards
The U.S. Food and Drug Administration took significant action to help increase the availability of ventilators and accessories, as well as other...
Blog: Regulations/Standards
On Wednesday, March 25, 2020 (3–4 p.m. Eastern), the U.S. Food and Drug Administration (FDA) will host a virtual Town Hall for clinical laboratories and commercial manufacturers...
Blog: Regulations/Standards
The Food and Drug Administration (FDA) has issued a new guidance to provide a policy to help expand the availability and capability of noninvasive remote monitoring devices to facilitate patient...
Blog: Medical
The U.S. Food and Drug Administration (FDA) has taken two additional significant diagnostic actions during the coronavirus outbreak (COVID-19) by...
Blog: Regulations/Standards
FDA is postponing most foreign and domestic inspections through April, effective immediately. Inspections outside the U.S. deemed mission-critical will still be considered on a...
Technology Leaders: Medical
Medical device manufacturers have a big challenge in preparing for their regulatory device submissions for 2020. In addition to the European Union’s new Medical Device...
From the Editor: Medical
FDA says it is continuing to advance its scientific and technical capabilities, but a recent New York Times editorial says the agency is in...
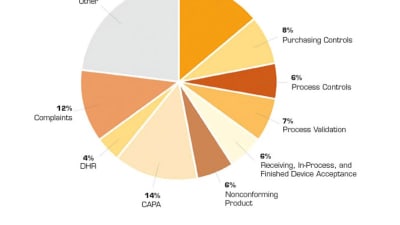
Briefs: Regulations/Standards
Medical device companies recognize that having an emphasis on design controls helps to assure good design and engineering practices that yield high-quality solutions. This is critical...
From the Editor: Medical
Under new rules to market medical devices in the European Union (EU), only 27 percent of respondents said they will be fully compliant with the regulations...
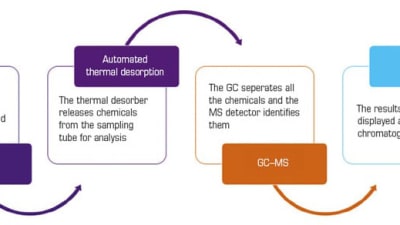
Briefs: Medical
In 2018, an update on regulations for respiratory medical devices meant that volatile organic compounds (VOCs) emitted from the devices...
From the Editor: Regulations/Standards
As a first step toward implementation of its recently established Safety and Performance Based Pathway for medical devices, FDA is issuing draft guidances outlining the...
Products: Wearables
Gradient Valves
Clippard, Cincinnati, OH, has released gradient valves that feature multiple two-way, normally closed solenoids connected around a central body. The NIV series...
From the Editor: Regulations/Standards
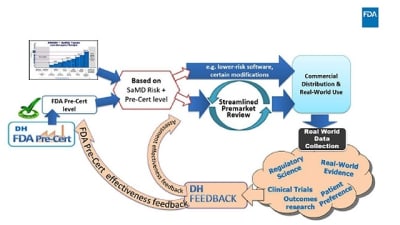
The test phase of FDA’s Software Precertification (Pre-Cert) Program has hit its halfway point. The agency has released an update on its progress and the lessons it has learned to date. The program aims to “reimagine” how FDA regulates digital health devices,...
Features: Regulations/Standards
Now that medical devices are being labeled and uniquely identified to meet the requirements of the U.S. Food and Drug Administration (FDA) Unique Device Identifier (UDI)...
Features: Design
Orthopedic devices play a crucial role in providing pain relief, improving mobility, and enhancing the quality of life for patients suffering from musculoskeletal...
Briefs: Design
While the regulatory and validation burden can be high, it's not surprising that the medical device industry is one of the earliest adopters of additive manufacturing (AM, aka 3D...
Features: Regulations/Standards
Companies regulated by the U.S. Food and Drug administration (FDA) need to establish current good manufacturing practices (CGMPs) as part of Title 21 CFR part 820 requirements. This includes...
From the Editor: Regulations/Standards
In the spirit of promoting public transparency, the U.S. Food and Drug Administration’s Center for Devices and Radiological Health is taking a number of...
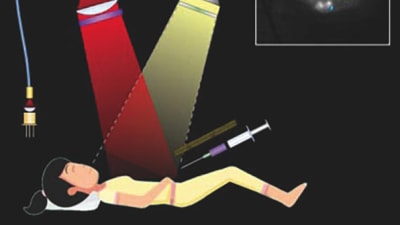
Briefs: Imaging
Ovarian cancer is usually diagnosed only after it has reached an advanced stage, with many tumors spread throughout the abdomen. Most patients undergo surgery to remove as many of these...
From the Editor: Medical
Spurring Innovation of Brain-Computer Interface Devices FDA is continuing to encourage innovation in novel areas — the latest being novel brain implants, often referred to as brain-computer interface devices — that can help patients with paralysis or amputation gain...
Features: Test & Measurement
The Luer connector is arguably one of the most important developments in the biomedical industry. Because it is reliable, simple to use, and economical to produce, the Luer connector...
News: Connectivity
The U.S. Food and Drug Administration (FDA) issued a safety communication to alert healthcare providers and patients about cybersecurity vulnerabilities identified in a wireless telemetry technology used for communication between Medtronic’s implantable...
Features: Regulations/Standards
The myriad of devices used in surgical, interventional, imaging, diagnostic and therapeutic, sensors, and single-use medical applications use some form of transmission medium to...
From the Editor: Software
When digital devices and technologies became the rule rather than the exception, FDA had to rethink its regulatory framework and evaluation process for...
From the Editor: Medical
Making changes to a regulatory process is usually difficult, slow, and often painful — after all, it involves lots of bureaucracy and many “cooks in the kitchen.” But...
News: Test & Measurement
As of September 2018, Instron’s Calibration Laboratory in Norwood, MA USA (NVLAP Lab Code 200301-0) expanded its global reach to include Europe into its existing operations. This addition...
Features: Electronics & Computers
Meeting the requirements for patient-connected medical devices can present challenges for power system designers. There are several key elements to consider, including isolation,...
News: Regulations/Standards
On October 10, 2018, FDA will host a webinar to share information and answer questions about the Quality in 510(k) (“Quik”) Review Program pilot. More information about the webinar is available here. On September 6, FDA launched the “Quik” Review Program...

 Podcasts: Medical
Podcasts: Medical Podcasts: Design
Podcasts: Design Podcasts: Design
Podcasts: Design On-Demand Webinars: Medical
On-Demand Webinars: Medical Podcasts: AR/AI
Podcasts: AR/AI Podcasts: Manufacturing & Prototyping
Podcasts: Manufacturing & Prototyping