The third edition of IEC 60601-1 has been in effect since June 2012. It replaces the previous version as a basic standard for medical electrical equipment and describes the general requirements for basic safety and essential performance features. The power supply unit can be considered as a component in this system and used, for instance according to EN 60950-1, in medical electrical equipment as an appropriate authorization. It is the MOP (Means of Protection) used for medical device requirements to protect both the operator and the patient from electric shock.
After the third edition of IEC 60601-1 for medical electrical equipment came into effect in June 2012, there are a few changes which must be considered in the design of the overall medical system as well as the power supply. The essential requirements represent an influence on the design of the safety point of separation listed below.
The general approach, like an electric shock caused by a faulty electrical equipment, may be avoided for the user, based on an integrated safety philosophy and goes beyond the scope of IEC 60601-1. The basic approach consists of two independently functioning safety mechanisms, referred to as a standard user protection. IEC 60601-1 distinguishes this protection in the operating personnel MOOP (Means of Operator Protection) and protection for the patient MOPP (Means of Patient Protection). The required user protection depends on the type of medical electrical equipment and must be specified by the manufacturer for each product and its purpose within the meaning of this standard.

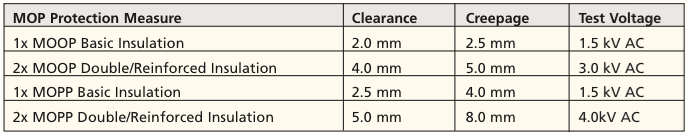
From early in the development phase, the design engineer must consider the demands on the power supply, in relation to the test voltage and the required clearance and creepage distance to the transformer and the circuit board. Table 1 shows the minimum distances for the realization of the protection, but the routes mentioned only have a theoretical value. Actual results, as shown in Table 1, lead to larger values.
A practical example for the design of clearances and creepage distances of a power supply at the interface between primary and secondary side with a 2 x MOOP protective measure is intended to illustrate the path of development within 60601-1-3rd. The main operating voltage is 230V AC, the converter topology is selected, a first circuit board layout is present, and the performance sufficiently defined. To obtain the clearances and creepage distances, the procedure is as follows. First, the maximum operating voltage is applied to determine the respective insulation (in the transformer and on the circuit board). This is done by using a scope, in order to obtain both the RMS and the peak value of the voltage with respect to insulation. It is measured on adjacent bare copper conductor paths, or connections of components, e.g. the transformer, optocoupler, Y capacitors, etc., and is determined at the interface between the primary and secondary side. The measurement results at the transformer terminals will show an effective value of 265V and a peak of Û = 552V. With these data, the clearance and creepage distances can be determined by applying the relevant data from tables in the 3rd edition as follows.
Interpretation of the clearance: From Table 13 in the 3rd edition, we found using 265V rms, a clearance for 2x MOOP of 4.0mm. In addition, based on the measured peak value of Û = 552V, an additional clearance of 0.4 mm is added using Table 14 in the 3rd Edition for 2x MOOP, resulting in a total clearance of 4.4mm. The device specification allows for use up to an altitude of 4,000 m. This requires a further enlargement of the clearance with a correction factor of 1.29 (from Table 8 of the 3rd Edition). The net required clearance from this amounts to (4.0 + 0.4) x 1.29 ≥ 5.7mm.
Interpretation of the creepage distance: Using 265V rms, pollution degree 2 and a CTI (Comparative Tracking Index) value of IIa or IIb is a creepage distance of 3.2 mm for 1x MOOP (see Standard Table 16). For 2x MOOP this means 2 times the value, or the use of a protective conductor already providing a degree of protection. For example, in a power supply with a protective conductor, the required creepage distance is 3.2mm. However, this is only theory, because the third edition leaves nothing to lower creepage but clearance.
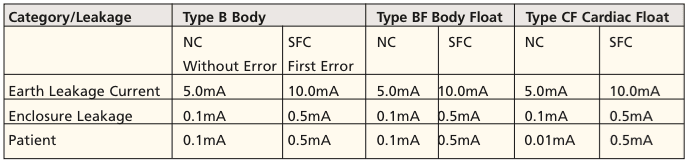
In practice, the values of Type B apply where power supply values from Tables 1 and 2 are tested to EN 609501-1, and can be used for powering electrical medical equipment. It should be noted at this point that the power supply is only considered in the overall medical system as a tested component. The power supply supplier is therefore not required to carry out a risk assessment procedure as mentioned above, since the manufacturer of the medical device, must make this risk assessment anyway. The design engineer of the power supply should offer good advice during the development of an appropriate FMEA (Failure Mode Effects Analysis) schedule and execution. The medical device manufacturer should seek approval of the components prior to their use in its medical electrical device, to meet the demands placed on it by risk management in terms of the standard.
The above example makes it clear that the requirements of both the electrical and the mechanical properties, such as the size of the power supply, have been determined safe for medical applications. The aforementioned risk analysis is used to obtain an effective means of providing the proof of protection concept.
This article was written by Reinhold Schulz, technical writer for GlobTek, Northvale, NJ (translated from the original German). For more information, Click Here .



