Briefs: Design
Neither the MDR nor Annex XVI are new. Nevertheless, the entry into force of the common specifications now presents a new situation for manufacturers of devices without an intended medical purpose.
Blog: Regulations/Standards
Understanding what the FDA really means by their time frames, knowing how to address their regulatory issues, and preparing a response to a 483 or Warning Letter is critical to showing the FDA that you understand what they expect from you and that you will work to bring your company into compliance. Another critical point is to understand that you can negotiate with the agency.
News: Regulations/Standards
The U.S. Food and Drug Administration has created a new Digital Health Advisory Committee to help the agency explore the complex, scientific and technical issues related to...
News: Medical
To foster the development and commercialization of medical devices designed for children, the Food and Drug Administration (FDA) has awarded a nearly $7.5 million grant to...
From the Editor: Medical
Written by Katie Falcone, Scientific Support Manager, Datwyler, this article addresses the dangerous implications of micro material contamination.
Briefs: Regulations/Standards
The EPA issued two separate proposals earlier this year covering the use of EtO for device sterilization: the National Emission Standards for Hazardous Air Pollutants and the preliminary interim decision under the Federal Insecticide, Fungicide, and Rodenticide Act.
News: Regulations/Standards
The Performance Review Institute (PRI) has launched a new service designed to help companies improve manufacturing and quality management and improve business...
News: Design
Organ transplant developer, Paragonix Technologies has received FDA clearance for its next-generation donor lung preservation system, BAROguard™....
News: Manufacturing & Prototyping
The U.S. Food and Drug Administration (FDA) updated its Catalog of Regulatory Science Tools by adding four new tools and updating nine others. The catalog is a peer-reviewed resource...
News: Regulations/Standards
TÜV SÜD has received approval as a UK Approved Body (UKAB) for medical devices. With this approval, medical device manufacturers can implement UKCA certification for the UK market and CE...
Briefs: Regulations/Standards
Thanks to artificial intelligence (AI), augmented reality (AR) has long shaped product development across a variety of areas, including the medtech industry.
Supplements: IoMT
In our summer edition of the MDB Resource Guide, learn about new cleaning requirements and find the right company to match your medical-design needs.
News: Medical
An agreement reached by the UK and EU regarding the Windsor Framework brings changes to the Northern Ireland regulatory landscape. The agreement , in principle, was reached on...
News: Test & Measurement
The current U.S. regulatory framework for medical devices under the Center for Devices and Radiological Health (CDRH) includes extensive...
News: Manufacturing & Prototyping
The Performance Review Institute (PRI) has expanded its management systems certification portfolio through the acquisition of SRI Quality System Registrar. SRI is headquartered...
Features: Design
The Institute for Engineering in Medicine’s Innovation Week highlights events that focus on advances and new directions in medical engineering.
Trivia: Regulations/Standards
It's March Madness! What organization was founded by a small group of physical educators and physicians who recognized that health problems were associated with certain lifestyle choices, especially smoking and lack of exercise?
News: Medical
Movano Health, Pleasanton, CA, has announced successful preliminary results of its pivotal hypoxia trial, which was completed in conjunction with the...
Supplements: Regulations/Standards
In our summer edition of the MDB Resource Guide, learn about new cleaning requirements and find the right company to match your medical-design needs.
Features: Software
Medical device manufacturers face constant changes coming from both internal and external sources.
Trivia: Physical Sciences
In November 1848, what was the first medical school to open for women?
Trivia: Physical Sciences
In July 1965, what landmark amendment was passed that helped drive medical innovation and improve the quality of healthcare?
Trivia: Regulations/Standards
At the turn of the 19th century, what industry was so dangerous that surgeons became specially trained to address the unique injuries of its employees?
Supplements: Sensors/Data Acquisition
In our summer edition of the MDB Resource Guide, learn about new cleaning requirements and find the right company to match your medical-design needs.
Features: Regulations/Standards
Although the pandemic has delayed the MDR’s validation date, it has not diminished the importance of this new regulation.
Technology Leaders: Materials
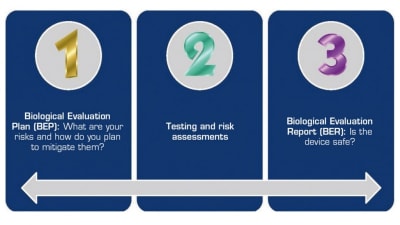
Modern evaluation of biocompatibility uses a risk-based approach.
Features: Medical
Bring your project from the process development phase of manufacturing to a fully validated one.
From the Editor: Connectivity
New best practices for understanding medical device security.
Supplements: Mechanical & Fluid Systems
In our winter edition of the MDB Resource Guide, learn about new cleaning requirements and find the right company to match your medical-design needs.

 Podcasts: Medical
Podcasts: Medical Podcasts: Design
Podcasts: Design Podcasts: Design
Podcasts: Design On-Demand Webinars: Medical
On-Demand Webinars: Medical Podcasts: AR/AI
Podcasts: AR/AI Podcasts: Manufacturing & Prototyping
Podcasts: Manufacturing & Prototyping