
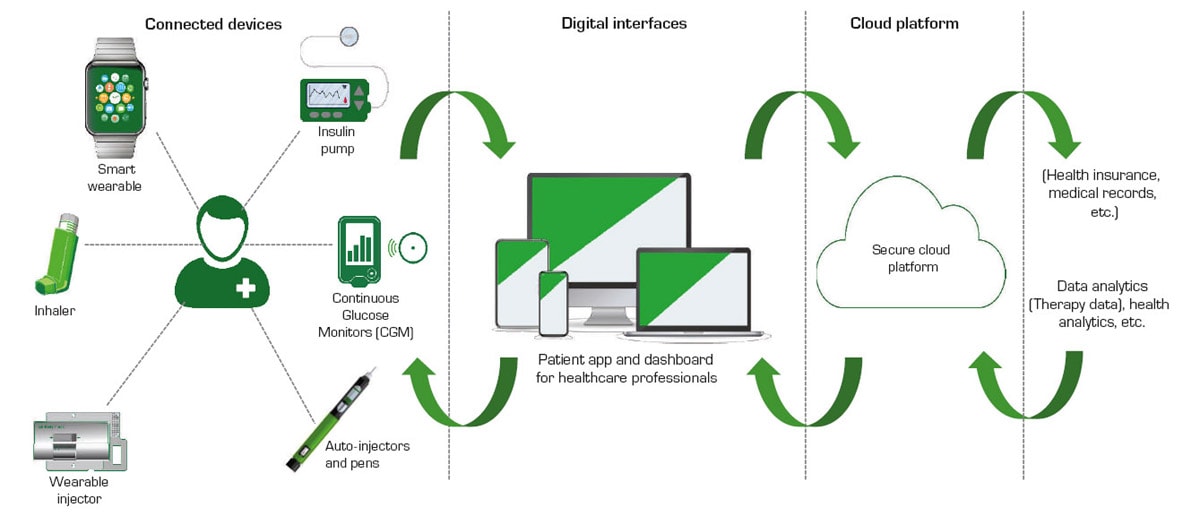
Advances in IoT and electronic technology are enabling more personalized, continuous medical care. People with medical conditions that require a high degree of monitoring and continuous medication infusion can now take advantage of wearable medicine injection devices to treat their problems. Wireless communication allows medical personnel to monitor and adjust the amount and flow rate of an individual’s medication. The small size of the injectors enables the individual to be active and not be burdened or limited by a line-powered instrument (see Figure 1).
Designers must develop a product that operates reliably 24 hours a day, seven days a week. The product must also be as efficient as possible to maximize battery run time between charges. With space and weight at a premium, designers must minimize the number and size of components while incorporating all the essential functionality and ensuring robust operation.
This article provides designers with recommendations for circuit protection against electrical hazards such as overcurrent and electrostatic discharge (ESD). It will also introduce designers to some unique sensing and detection components that enable low-power circuit design. These components will allow designers to achieve robust, reliable performance in a small, lightweight, low-power product.
Description of a Wearable Medicine Injector
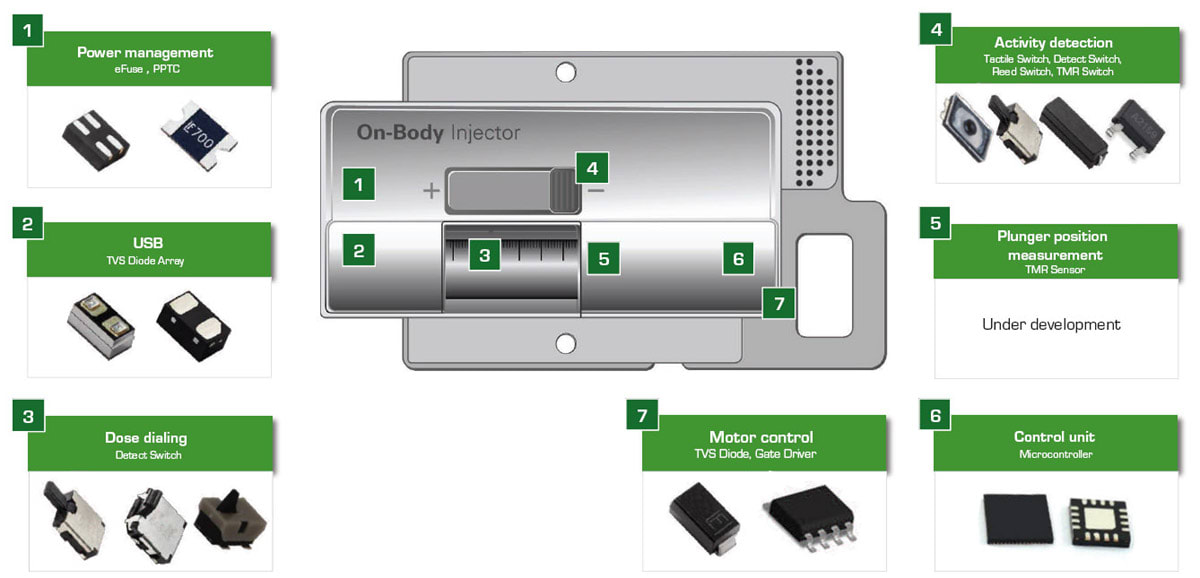
Figure 2 illustrates a wearable medicine injector. Surrounding the injector are blocks indicating the components that provide protection and efficient control for the medicine injector’s circuitry. Figure 3 presents a block diagram of a wearable medicine injector. The table on the right of the block diagram lists protection and control components recommended for use in specific circuit blocks.
A long-life lithium-ion (Li-ion) battery powers the medicine injector. A USB source provides power to charge the battery. Depending on the power requirements of the injector, recharging the battery will require either a USB-A interface, a USB-B interface, or a USB-C interface. The USB-A and -B interfaces can provide up to 20 W of charging power, while the USB-C interface can deliver up to 100 W.
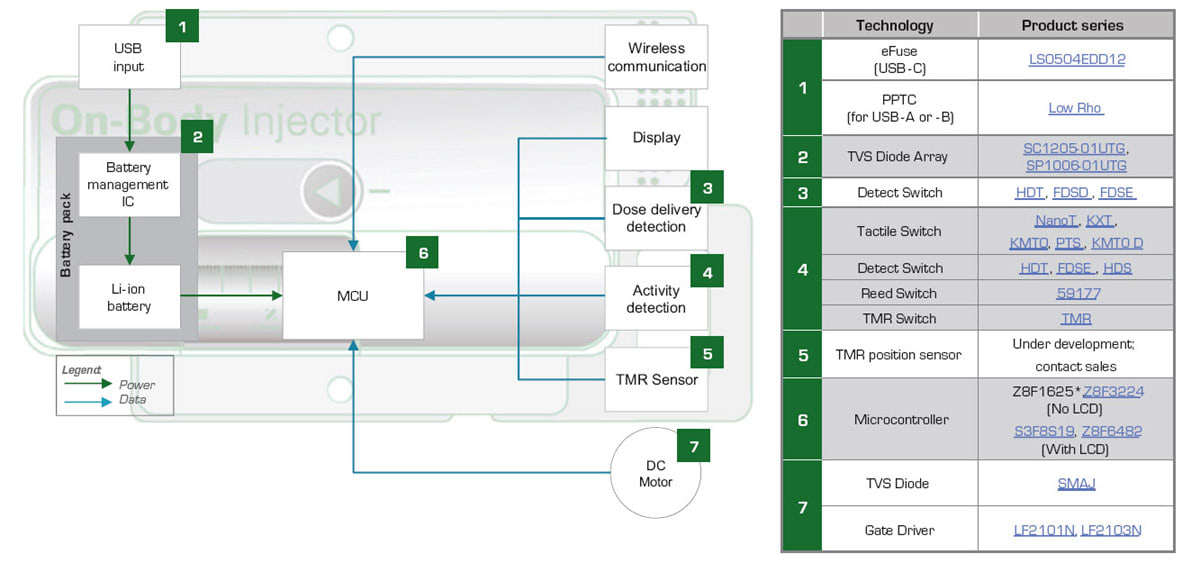
The microcontroller unit (MCU) drives the injector motor and obtains position feedback from activity detection and position sensors. The injector transmits data and receives adjusted settings from medical personnel through a wireless communication interface.
Robust Protection
Robust operation requires protection from electrical hazards. Using special-purpose components will withstand the hazards to protect the circuit blocks. If using a USB-A or -B input protocol, a polymer positive temperature coefficient (PPTC) resettable fuse provides overcurrent protection of the USB Input circuit. Look for PPTC fuses with very low resistances to minimize power loss through the fuse. Models of PPTC fuses, for most current ratings, have internal resistances under 1 Ω. PPTC fuses are also available in small, space-saving surface mount packages.
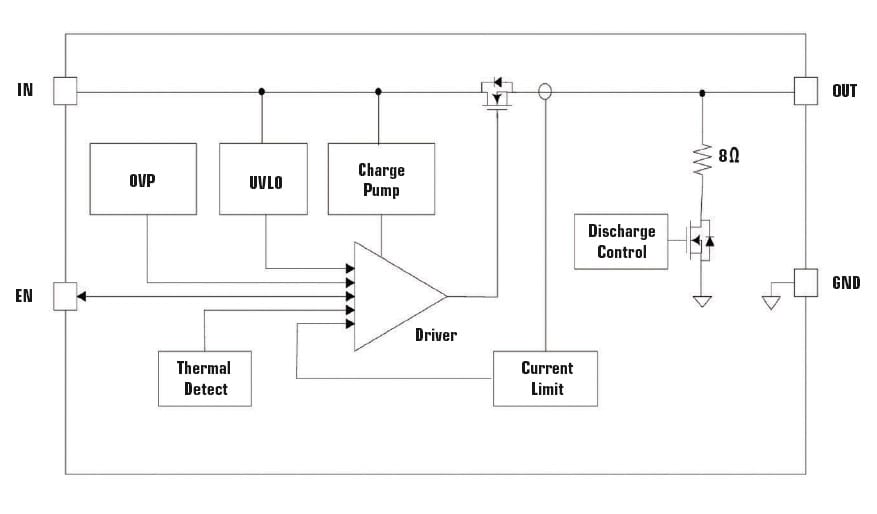
A more complete USB input circuit protection alternative for the higher power USB-C interface is a one-chip solution, a 4-pin surface-mounted integrated circuit (IC) that combines overcurrent, overvoltage, and overtemperature fault protection. This component, whose block diagram is shown in Figure 4, is one model in the family of Littelfuse products known as eFuse™ circuit protection ICs. In addition to protection against overcurrent, overvoltage, and overtemperature fault conditions, an eFuse has soft-start circuitry to minimize inrush current and under voltage lockout to prevent operation until the supply voltage has reached an adequate level. To minimize power loss, the eFuse has a series load switch FET with very low on-resistance of 26 mΩ. If any fault conditions are detected, the load switch FET drops the input voltage to a level that shuts down the injector. This eFuse fits in a space-saving DFN1.2×1.6_4L surface mount package.
The battery management IC monitors the battery’s state-of charge (SoC) and state of health (SoH) to maximize battery life. Maintaining a reliable circuit requires protection from ESD. A transient voltage suppressor (TVS) bidirectional diode array containing two TVS diodes connected anode-to-anode can safely absorb ESD strikes as high as ±30 kV. Models of TVS diodes typically respond in nanoseconds and clamp the voltage to 10 V to protect the battery management IC.
The motor drive circuit needs protection from power surges and ESD. A TVS diode protects the circuit from both hazards. Available surface-mount components can safely absorb up to 400 W of surge pulse power and 30 kV ESD strikes. These TVS diodes have an ultra-fast response of under 1 ps to transients. There are two configurations: a unidirectional single-diode design and a bidirectional cathode-to-cathode device.
TVS diodes can also protect the display and wireless communication circuits from ESD. Low capacitance TVS diodes can minimize distortion of low-level RF signals to avoid injection of errors in received signals. This small number of components is all that is needed to protect the circuity from electrical hazards. The components consume minimal power, and they have space-saving, surface-mount packaging.
Optimized Efficient Sensing and Control
Minimizing power consumption and space requires efficient, small sensing and control circuit blocks. The options described in the following paragraphs will assist the designer in achieving these two design goals.
The dose delivery detection circuit requires a switch to indicate the position of the dose dial. Surface-mount detect switches that are as small as 3.5 mm wide × 2.8 mm deep × 3.35 mm high and have a low activation force of 35 g are available. These switches have a low contact resistance of 500 mΩ and a life exceeding 100,000 cycles. Designers can select top or side activation.
The activity detection circuit performs several functions, including safety indications of the presence of the drug vial and verification that the injector output is in contact with the patient’s skin. This circuit also activates drug injection and performs drug dose monitoring. Designers have options for detecting these conditions.
One option for activation is a small low profile, top-actuated, surface mount pushbutton switch. Dimensions are 2.5 mm wide × 1.65 mm deep × 0.55 mm high. Switches are rated IP67 against ingress of moisture and dust. Pushbutton switch life cycles can be as high as 300,000 cycles and contact resistance is a low 500 mΩ. A second option is the type of detect switch suggested for the dose delivery detection circuit.
A third option is the use of a reed switch. Designers can specify ultraminiature, hermetically sealed reed switches with variable sensitivities. Magnetic actuation does not draw any power from the circuit, and the current leakage through the switch is at negligible pA levels.
A fourth option is a tunneling magnetoresistance (TMR) switch. It consists of a magnetic tunneling junction sensor and CMOS circuits. This magnetically triggered digital switch combines high sensitivity and ultra-low power consumption. Figure 5 shows a block diagram of a TMR switch. A temperature compensated regulator circuit ensures voltage stability for the internal circuits. A Schmitt trigger provides switching hysteresis for noise rejection, and a CMOS push-pull circuit provides the output drive.
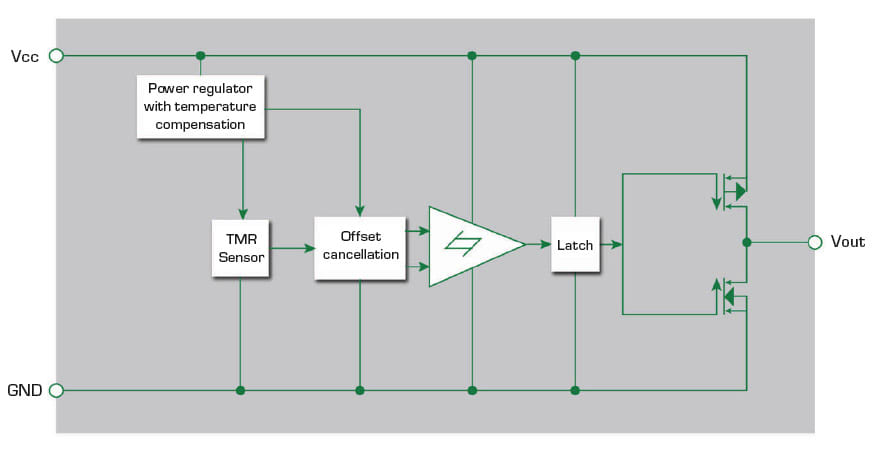
The TMR switch is next to the bottom of the injector’s plunger chamber, and a small magnet mounts on the injector’s plunger. As the motor pushes the plunger toward the bottom of the injector and the TMR switch, the switch produces an increasing output voltage and switches logic state. The switch indicates the plunger’s position. The microcontroller (MCU) uses the position information to determine the volume of the injected drug volume.
The TMR switch draws only 200 nA when powered by 1.8–5 V circuits. Its sensitivity can detect a magnetic field as low as 5 Gauss. The CMOS output enables direct connection to an MCU which avoids the need for additional components. The high sensitivity and low power minimize battery drain, making a TMR switch a superior solution for wearable injector drug dose monitoring.
Control of the injector requires a microprocessor or a microcontroller. To save space and power, single-chip MCUs can provide all the necessary functionality and draw low power. MCU sleep modes can significantly lower power draw during periods of inactivity.
High-efficiency 8-bit MCUs are available for control of injector operation. Available MCUs can have operating current as low as 2 mA. Lower clock speed settings can reduce the operating current even further. These MCUs have low-power inactivity modes that reduce current consumption to 0.7 μA. Models can have 15 analog-digital converter channels with 12-bit resolution, 16 Kbyte flash memory, and an on-chip temperature sensor array. These MCUs have high resolution timers for PWM drive of motors.
They also have peripheral I/O ports optimized for interfacing with wireless communication chipsets. For efficient control of the motor, use a gate driver to interface between the MCU and the power MOSFET or IGBT motor drives. Gate drivers have TTL and CMOS inputs to allow direct connection to a MCU output control line. Schmitt triggered logic inputs minimizes motor torque ripple, and 50 ns propagation delay between high and low side outputs enables precise control of the motor.
Standards for Wearable Medicine Injectors
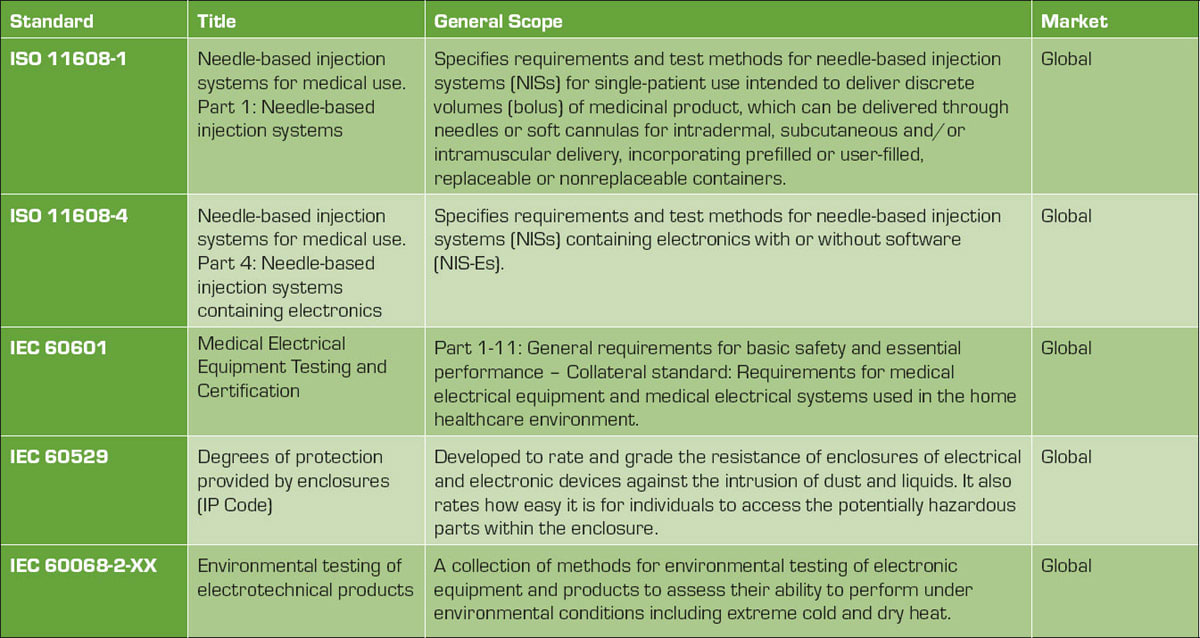
Medical devices are highly regulated for the purpose of patient safety. Table 1 lists the global standards design engineers must adhere to for development of a compliant device. Understanding these standards and their impact on device design is essential for obtaining medicine device approval from the regulating bodies.
Expertise in Robust and Efficient Design
Ensuring reliable performance is essential for wearable medicine injectors, which must operate 24 hours a day, seven days a week. Protecting an injector from hazards such as overcurrent, overvoltage, overtemperature, and ESD is critical for ensuring reliable, robust operation. Adding components that provide circuit protection counters the requirements for small device size, low power consumption, and low weight. Surface-mounted, low-power consumption components introduced in this article can minimize the impact of achieving circuit protection while not significantly impacting size, power, and weight targets.
Designers can save development time and compliance costs by taking advantage of component manufacturers’ expertise. Their application engineers can help with the selection of cost-effective and efficient protection and control components. In addition, they can help guide design requirements dictated by the applicable standards. Some manufacturers, such as Littelfuse, can perform precompliance testing to avoid compliance test failures, saving testing costs and avoiding delays due to multiple compliance test cycles. Expert assistance combined with the recommended components will result in a low-power and robust, small, lightweight wearable medicine injector.
This article was written by Dr. Marco Doms, Sr. Manager Business Development New Platforms, Littelfuse, Inc., Chicago, IL. He is responsible for several platforms with new products or product features that require additional internal and customer coordination. For more information, visit here .



