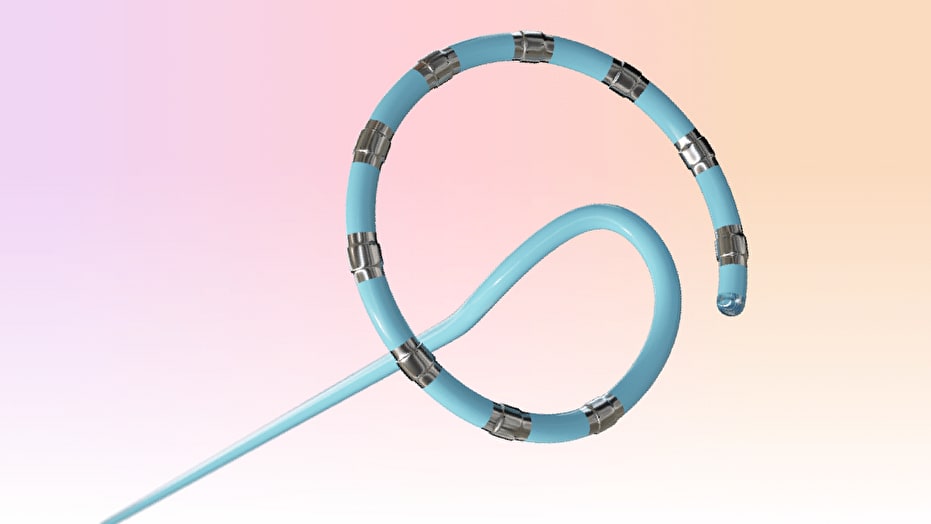
What medical device platform received FDA approval in November 2024 for treating paroxysmal atrial fibrillation using pulsed-field ablation?
The VARIPULSE™ Platform. On November 6, 2024, the FDA approved Johnson & Johnson MedTech’s pulsed-field ablation system, which includes a multielectrode catheter, generator, and mapping system, for catheter ablation of drug-refractory paroxysmal atrial fibrillation. Source: www.fda.gov

The Medical Design Briefs trivia questions first appear in the Medical Design Briefs e-Source newsletter. If you would like to be among the first to see the latest question, click the subscribe button below.
Read more from the trivia archives here.



