The COVID-19 pandemic has expanded the public’s awareness of health-related issues. Precisely targeted technological methodologies and devices that can solve specific healthcare problems are becoming increasingly important for medical applications. Utilizing microelectromechanical systems (MEMS) technology, German microsystem technology R&D firm Hahn-Schickard developed an efficient medical-device sterilization cycle counter. Being able to autonomously record their life cycles helps medical instruments protect patients’ safety. Moreover, this capability simplifies hygiene management in hospitals, clinics, and doctors’ offices since no additional activity is needed to record individual sterilization cycles.
The project revolved around a simple but important specification: the maximum allowable number of steam sterilization cycles for reusable medical devices. Since the sterilization process requires exposure to extreme conditions of relative humidity and temperature, any device designed to operate in these conditions needs to be both robust and impervious to degradation over time. This environment would prove challenging for most conventional electrical sensing devices.
The current method of manufacture for this type of MEMS device involves bonding three wafers (in this case, 100 mm in diameter) in a layered stack, using separate buffered oxide etch (BHF) wet-etching steps for both the top and bottom glass wafers, as well as multiple associated processing steps. This is neither an elegant nor an efficient solution to realizing such a device.
A Simpler Process
Hahn-Schickard developed a greatly simplified, single-step vapor hydrogen fluoride (v-HF)-based micromachining manufacturing process that eliminates the need for the bottom glass wafer. The process uses a memsstar ORBIS 3000™ tool that includes both v-HF and an anti-stiction self-assembled monolayer (SAM) coating process chamber to control moisture variation.
With this configuration, multiple connected silicon dioxide (SiO2) or glass layers can be etched in a single process step, and a final hydrophobic SAM coating can be applied, without a vacuum break, to prevent stiction or device reliability issues. This means that the device will guarantee reliable long-term performance in a high-humidity environment. The other key benefits of a single-wafer processing tool like the ORBIS 3000 are that the within-wafer uniformity can be repeatably controlled, and the process is not subject to the wafer-to-wafer uniformity issues observed with batch processing tools (which are further compounded by varying batch sizes).
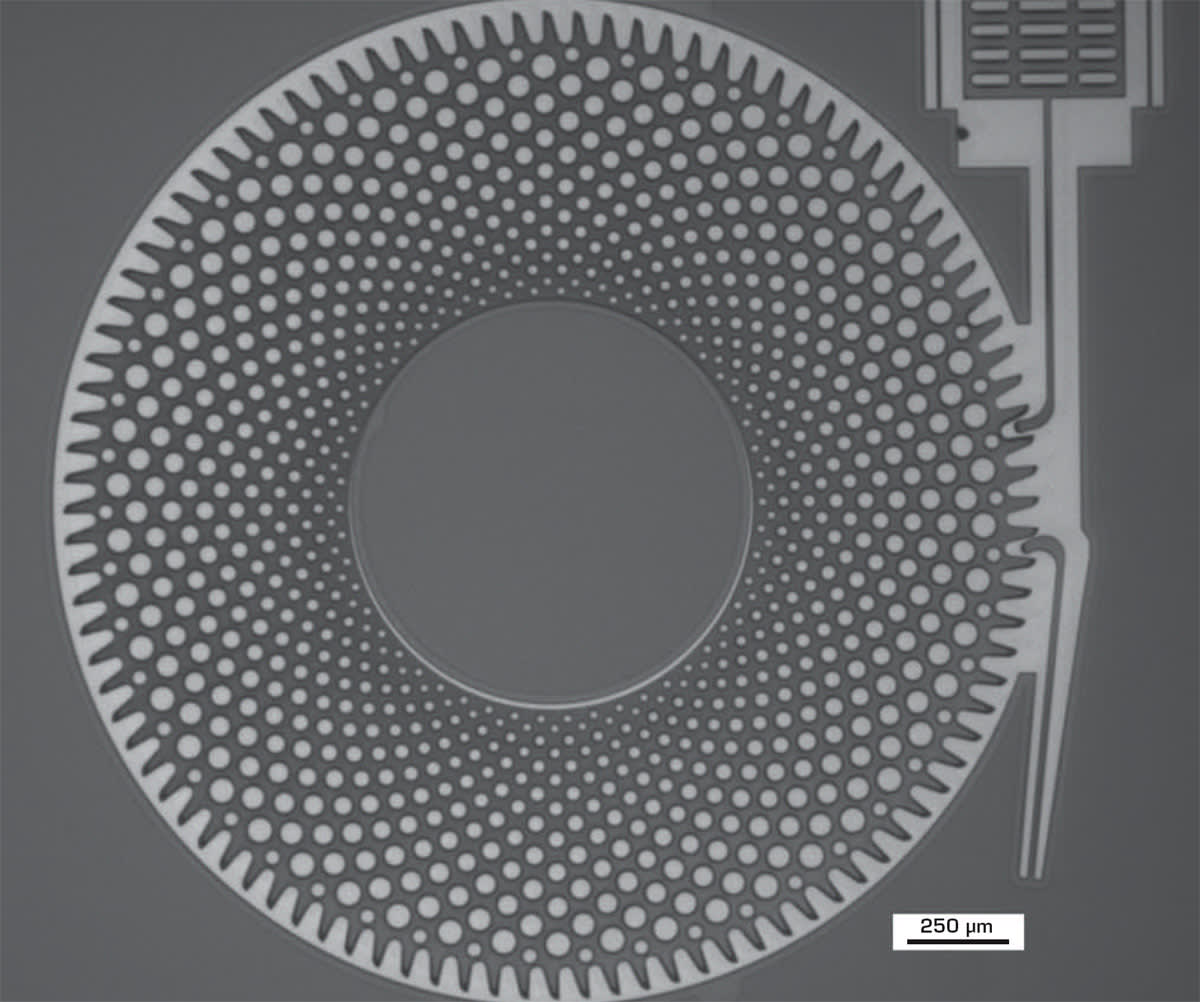
To develop the new MEMS sensor devices, Hahn-Schickard first designed a now-patented MEMS device with a moveable shuttle, gearwheel, and driving and escapement rods. These devices were then fabricated with a surface micromachining process using a 2-μm oxide silicon-on-insulator (SOI) wafer substrate with moveable silicon component parts. The device was perforated with a resist-patterned silicon deep reactive ion etching (Si DRIE) process, allowing access for a single-step v-HF release process to realize the functioning device. (Note: A hydrophobic SAM coating using perfluorodecyltrichlorosilane (FDTS) can be optionally applied to ensure that the device is repeatably fully stiction free.)
The device operates with a shape memory alloy (SMA) wire bending activated by a phase change over the temperature range of the sterilization cycle, which moves the push rod to the next tooth of the gearwheel, thus counting one sterilization cycle. Different design variants of the gearwheel were also designed and tested with an on-wafer simulated sterilization temperature cycle using a hot plate.
Experimental Procedure
The v-HF process used by Hahn-Schickard was developed by completing an initial screening experiment, followed by a full three-level factorial design of experiments (DOE) for HF flow and process pressure over two chuck temperature blocks of 5° and 25 °C. A full factorial DOE is a simple systematic design style that allows for estimation of main effects and interactions. The etch rate and uniformity were established by a 10-point measurement of the oxide undercut over the device wafer to calculate the etch rate for a fixed etch time. The uniformity was calculated as half the range of the etch rate divided by the average etch rate, expressed as a percentage. The DOE was repeated three times to improve the statistical significance of the results.
The process had an etch rate range between <100 and >900 nm/min and uniformities varying between 70 and 95 percent. The uniformity shows more temperature dependence at 25 °C. A chosen optimal process at 25 °C used a high HF flow and high process pressure and yielded an etch rate of 900 nm/min and 95 percent uniformity. Higher pressure improves both the etch rate and uniformity. Aluminium (Al) is present on the devices for labeling, and this does not appear to be damaged by the v-HF processing. The fully released device gearwheel with a 20-μm undercut beyond the Si contours is shown in Figure 1.
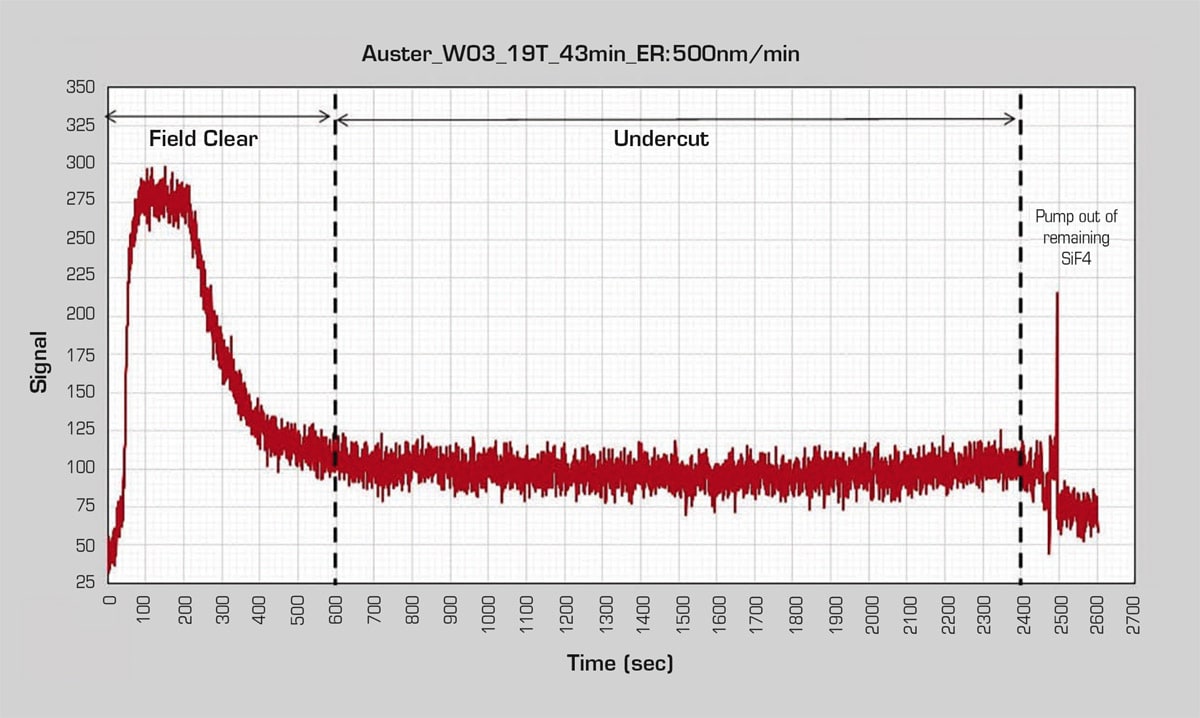
The ORBIS 3000 tool used by Hahn-Schickard allows effective process monitoring of any v-HF etch process on the memsstar ORBIS or ALPHA ™ process chambers. The tool was equipped with a nondispersive infrared (NDIR) process etch monitor, which can be used not only to monitor the progress of the etching process but also, in some cases, to control an end-pointed process. In this case, a typical undercut process etch monitor trace was observed where there was an initial field oxide etch step with a strong silicon tetrafluoride (SiF4) signal, which took about 10 minutes to complete. Next came the longer undercut etch to release the device, which took around 30 minutes to complete. The result achieved was the desired 20-μm oxide undercut (see Figure 2).
Conclusion
A medical instrument equipped with a device such as the one Hahn-Schickard developed utilizing the memsstar ORBIS system can record the number of sterilization cycles individually, and the MEMS sterilization cycle counter operates without the need of batteries. The function of the device has been verified within an autoclave demonstrating the feasibility of the concept. Current work focuses on manufacturing aspects in order to obtain robust counter devices with reproducible characteristics.
This article was developed from a paper presented at MikroSystemTechnik Kongress 2021, November 8–10, 2021, Stuttgart-Ludwigsburg. For more information, visit here .



