Noxilizer has developed a room temperature sterilization process for medical devices that is based on nitrogen dioxide (NO2) gas. Currently, industrial sterilizers and contract sterilization services using NO2 gas are being offered to medical device manufacturers for terminal sterilization during the manufacturing process. A hospital model is also being developed for use in the clinical setting as a means of sterilizing reusable medical devices. As such, designers should be aware of this technology today for disposable and implantable devices, and also in the future for reusable devices sterilized in hospitals using NO2 gas sterilizers. At present, the terminal sterilization market for medical devices is primarily serviced by ethylene oxide (EO) gas or radiation processes, with gamma accounting for the majority of radiation sterilization. In this article, NO2 sterilization is discussed in the context of other gas sterilization processes, but it may also be applicable to devices that are sterilized with gamma.
All gas sterilizers face challenges in addressing the wide variety of medical devices that are being produced today, or will be developed in the future. The first challenge is the fact that the gaseous sterilant needs to contact all of the device surfaces that require sterilization. There fore, the packaging and device design must permit gas access. For the device, this means avoiding closed spaces and mated surfaces. For the packaging, gas access is typically achieved through the use of porous packaging that provides a sterile barrier for the finished device. Tyvek® has been an industry standard in sterile barrier packaging for years because it allows ready gas diffusion in and out of the package. Tyvek pouches are compatible with EO, hydrogen peroxide (H2O2), steam, and NO2 sterilization. Additionally, EO can diffuse through polymers in order to sterilize within non-porous polymeric packaging. Sterilant access to all device surfaces can be ensured during the design process by allowing for paths through which the NO2 can flow, either under vacuum or by diffusion, into complicated geometries such as lumens and mated surfaces. With H2O2 (boiling point, Tb = 150 °C) condensation of the sterilant can occur prior to reaching the innermost regions of a device as the sterilant concentration approaches the saturated vapor pressure. This condensation can result in localized sub-lethal conditions leading to non-sterile devices. With NO2 (Tb = 21 °C) this is not an issue because of the high saturated vapor pressure at the sterilization temperature. Diffusion into lumens occurs without the driving force for condensation. EO sterilization relies on diffusion of the gas without the aid of vacuum to reach complicated geometries, which results in longer cycle times. EO can sterilize closed volumes within devices via diffusion through polymers given long enough exposure times.

Unlike EO, most polymers are rather impermeable to NO2, particularly over the relatively short exposure required with NO2 gas sterilant. This makes aeration a faster process. Since NO2 has a vapor pressure of 1 atm at room temperature, aeration can be carried out using either vacuum-assisted air exchanges or a steady flow of air at ambient pressure through the sterilization chamber. The aeration process with NO2 sterilization is generally about 15 minutes in duration, and this is built into an overall cycle time that usually runs about 60 to 90 minutes for the large industrial sterilizers.
NO2 sterilant is supplied as a liquid, from which vapor is dosed into the chamber during the cycle. This is a space saving alternative to large gas cylinders. NO2, while a toxic gas, is nonexplosive and non-carcinogenic. At the end of the cycle, NO2 is removed from the exhaust gas via a scrubber system that is built into Noxilizer’s industrial sterilizers, which can allow an NO2 sterilizer to be vented safely, without releasing NO2. The spent scrubber material is a non-hazardous solid waste product (considered landfill-safe in the US) that can be disposed of in accordance with local regulations. This makes NO2 sterilization a safer and more cost-effective option to bring sterilization activities inhouse and make it a real part of the manufacturing process.

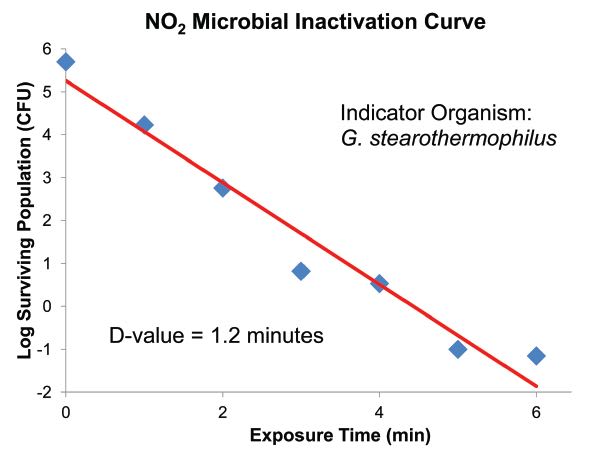
While temperature is not controlled, relative humidity (%RH) is a critical process variable. Lethality proceeds more rapidly with increasing humidity, but an SAL of 10-6 can still be achieved at reduced %RH with increased cycle duration. A cycle with 80%RH will typically require a sterilant exposure time of 20 to 40 minutes, while an exposure with 2 sterilization process. If %RH is not critical to device integrity, then the higher humidity cycles minimize the overall exposure to the NO2 gas.

As NO2 sterilization is not compatible with cellulosic materials like paper and cardboard, the sterilization step must be performed prior to final packaging. The product would be packaged in the sterile barrier packaging, sterilized with NO2, and then sent to final packaging, which may include paper inserts. This may represent a shift in manufacturing philosophy, but in order to realize cost savings or room temperature processing with NO2, it is one that is quite feasible particularly when sterilization is brought in-house. Procedural kits or devices that include a prefilled syringe represent an ideal candidate product for sterilization with Noxilizer’s NO2 process. The ability of NO2 to diffuse into packaging at ambient pressure allows the syringe to be sterilized without moving the stopper because no pressure is exerted upon it by the head space in the syringe. The low permeability of most polymers to NO2 means that the sterilant will not interact with, or contaminate, the drug product contained within the syringes. A short cycle time at room temperature reduces the likelihood of degradation of temperature-sensitive drug products. NO2 sterilization is compatible with many common syringe materials such as glass, cyclic olefins, polypropylene, silicone, most rubbers, and thermoplastic elastomers. In fact, NO2 can be used to sterilize syringe parts after manufacturing, as well as decontaminate syringe tubs prior to entering the filling line.
Most manufacturers prefer to use contract sterilization services for EO gas. The EO sterilization process is inherently lengthy due to the preconditioning, exposure, and aeration phases. The process time is often compounded with the time required to accrue sufficient inventory and to transport the product to the sterilizer. As a result, manufacturers can lose inventory to the sterilization process for several days up to one month, which adds inventory carrying costs to the equation. This factor seems to impact small- and medium-sized manufacturers the most, as they will not always produce enough to fill a commercial EO sterilization chamber and must wait to accumulate enough product to fill a chamber before their product is processed.
NO2 sterilization is a new option for medical device manufacturers. Designers of medical products can have the option of NO2 sterilization efficiencies through material selection and design choices. The advantage of the NO2 sterilization is in-house sterilization, which can save time and money by eliminating transportation and inventory carrying costs associated with contract sterilization. Man ufacturers are often reluctant to bring EO or gamma sterilization in-house because of the substantial capital investments and safety issues that are associated with the technologies. Gamma units are capital intensive, and require cooling water or other facilities modifications. Large EO units also require facilities modifications, such as abaters and explosion- proof walls, owing to the explosive and carcinogenic nature of the gas.
Noxilizer’s patented NO2 sterilization process provides a solution to some of the challenges and inefficiencies posed by other sterilization methods with the RTS 360 industrial NO2 sterilizer, shown in Figure 3. It is a self-contained unit (requiring only a power connection) that provides a usable sterilization load volume of 25" × 25" × 36". This is roughly equivalent to one fifth of a pallet of product. With a typical cycle time of 60 to 90 minutes, approximately one pallet of product can be sterilized in an eighthour shift. The product is safe to handle after the cycle and can be returned to inventory immediately. For manufacturers who wish to bring sterilization in house, this allows the sterilization step to become an integral part of the production line. The fast cycle turnaround of the RTS 360 is capable of supporting small, medium, and large companies’ lean manufacturing philosophies such as “just-in-time.” Noxilizer can also design and build custom in-line systems for manufacturers with high volumes that would see a greater financial benefit from a continuous sterilization process, as well as provide a contract service for NO2 sterilization.
This article was written by Evan Goulet, Ph.D., Technical Applications Manager, and David Opie, Ph.D., Senior Vice President of Research and Development for Noxilizer (Baltimore, MD). For more information, Click Here



