
To reduce hospital-associated infections caused by microorganisms, medical devices are typically cleaned and disinfected with chemical disinfectant solutions, including disinfecting wipes. Disinfectant use increased markedly during the COVID-19 pandemic. These disinfectant formulations tend to be comprised of an active ingredient, a solvent or aqueous carrier solution, and additional constituents that enhance the formulation’s properties.
Common active ingredients include quaternary ammonium compounds, sodium hypochlorite, and hydrogen peroxide. In some cases, disinfectant formulations are predominantly alcohol based with no other active ingredient and minimal additives. The carrier solutions are often aqueous in nature and frequently contain isopropyl alcohol or ethanol. The additional constituents are a myriad of chemical species that improve a disinfectant formulation’s performance and delivery. These additives can improve active ingredient efficaciousness, increase active ingredient stability, and alter wetting behavior of disinfectant solutions. However, some of these additives can have damaging effects to medical device external surfaces and enclosures, reducing the material’s mechanical properties. This is especially true for thermoplastic materials. Disinfectant manufacturers investigate the effects of chemical disinfectants on enclosures through material testing to reduce negative impacts. Disinfectant manufacturers are ultimately constrained to formulation choices that kill as many microorganisms as quickly as possible. The formulations need to be efficacious.
Plastic Enclosures
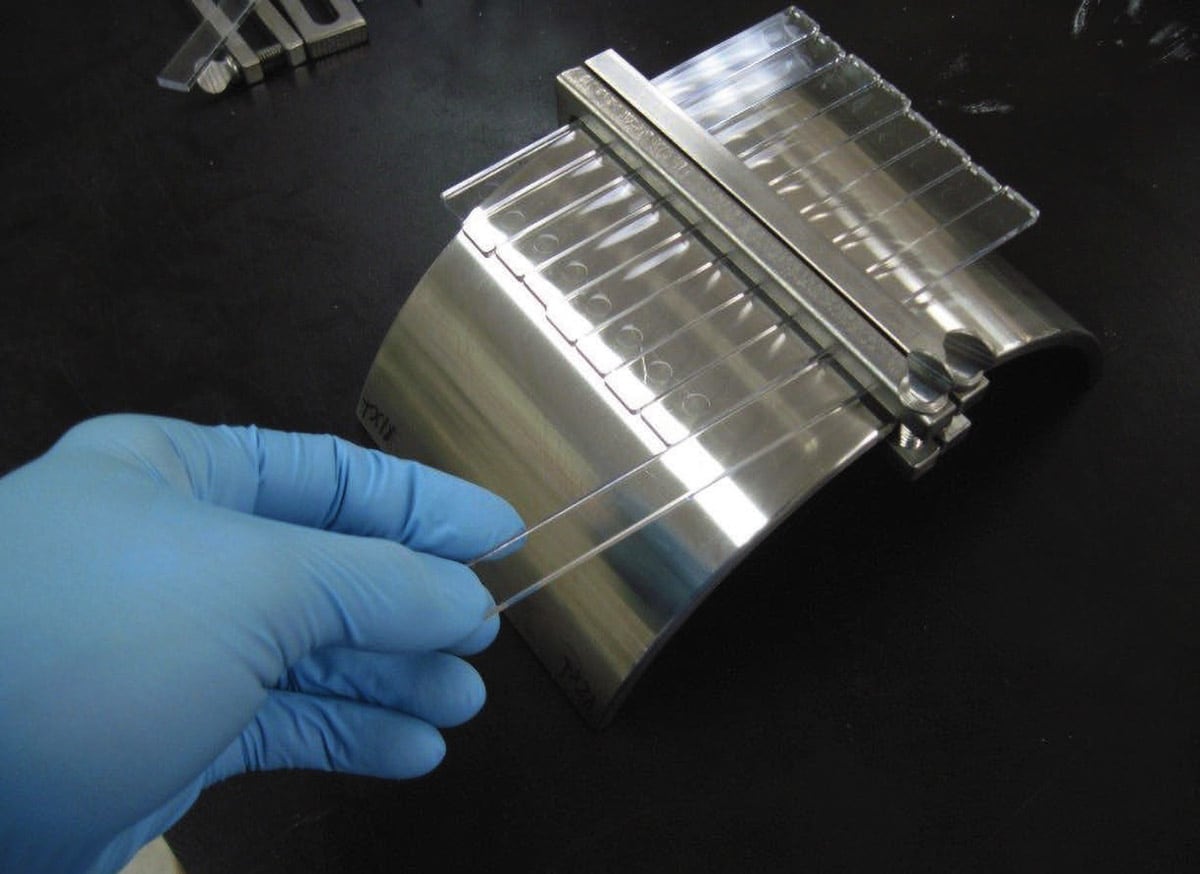
Raw material manufacturers (polymer/plastic companies) have the difficult task of producing plastics and plastic alloys that meet a host of property expectations for medical device enclosures. Plastic enclosures are expected to meet certain mechanical, electrical, and thermal properties while maintaining a percentage of these properties throughout the enclosure’s life cycle. Additionally, polymer formulations need to be workable resin systems, capable of ease of use at the point of manufacture.
A panoply of additives are integrated into polymer formulations to assist in the manufacturability of the resin systems. The final part products need to have some level of chemical resistance; this is especially true for medical device enclosures due to repeated exposure to chemical disinfectants. Like disinfectant manufacturers, raw material manufacturers rely on various testing methodologies to evaluate chemical resistance of their materials. This testing informs the raw material manufacturers about their product’s response to chemical disinfectants used in the healthcare space.
The companies that make medical devices, or original equipment manufacturers (OEMs), use thermoplastic materials to enclose and protect their devices, ensuring that they maintain full functionality when exposed to the environmental stresses seen in multiple use cases, while continuing to meet the device’s design criteria and safety requirements, as well as being aesthetically pleasing, ergonomic, and cost-effective.

Because these devices are frequently cleaned with chemical disinfectants, the polymer resins sourced need to be chemically resistant or compatible with a significant number of chemical disinfectants and solutions. Plastic materials incompatible with common chemical disinfectants can weaken, craze, crack, embrittle, exhibit leaching, and eventually lead to substantial enclosure breakdown. OEMs rely on material performance data to make well-informed decisions on enclosure materials. The greater the similarity in chemical resistance data across material specifications, the easier it is for OEMs to compare data and choose enclosure materials that best suit the end user’s needs. Medical device failure in the field has substantial adverse impacts to end users, reduces hospital efficiencies, and increases patient risk.
Selecting Chemically Resistant Materials
How does one select chemically resistant enclosure materials for medical devices? How are the materials tested for resistance to chemical disinfectants? Luckily, chemical resistance is not a new concept; there are a variety of ASTM and ISO material testing standards that guide material testing. Common testing standards are ASTM D 543, D 638, and D 6110. These standards are used across industries to guide material conditioning, exposure, and mechanical testing of materials.
Standards generally afford the experimenter some latitude in experimental design, often written to provide a broad framework in which to conduct the experiment. However, this broad framework can cause a lack of uniformity in conditioning, exposure, testing, and pass/fail criteria. Pass/fail criteria are often aligned to the experimenter’s own needs and expectations, further confounding data generated. This often occurs in chemical resistance testing/environmental stress crack testing, skewing results. Currently, no consistent minimum testing standards are required to validate that disinfectants are compatible with surface materials used for the design of medical devices.
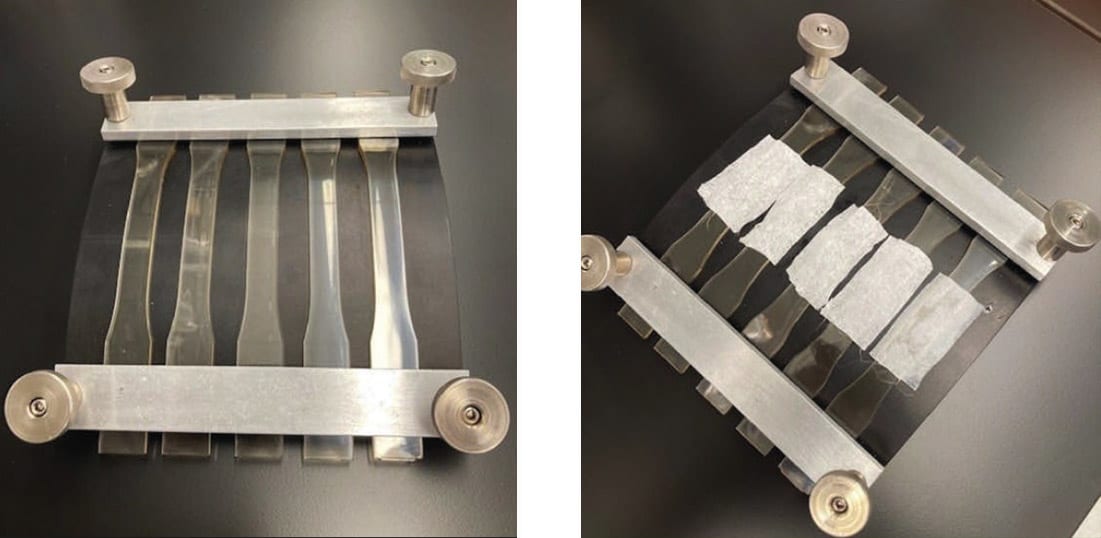
A common source for testing inconsistency comes when executing ASTM D 543, Standard Practice for Evaluating the Resistance of Plastics to Chemical Reagents. This standard provides the mathematical relationship between a tensile bar’s out fiber strain, the tensile bar’s thickness, and the radius of curvature that the bar is deflected against. This standard, in essence, helps the experimenter define geometry of strain jigs and affords the experimenter the freedom to determine the level of strain.
Several different strain values, sometimes inconsistently, are used as a result of experimenter freedom. An unfortunate result of this freedom is the plethora of strains used across a multitude of plastic materials throughout the medical device design and manufacturing space. Further convoluting test results are exposure times; the experimenter is free to choose the intervals for plastic exposure to chemical disinfectant. These differences in strain amounts and exposure periods make data comparisons across medical device plastics difficult. OEMs are often at a loss on how best to evaluate one material against another when selecting for chemical resistance. A consensus on clearer conditioning, exposure criteria, and pass/fail criteria that all chemical disinfectant manufacturers and plastic manufacturers follow would help obviate this problem.
Coming to Consensus
In the Spring of 2020, under the aegis of the nonprofit organization HealthCare Surfaces Institute (HSI), engineers and scientists representing different companies across the medical device and healthcare space met to work on the conditioning, exposure, and testing of plastics used for medical device enclosures. The participants formed the Plastic Testing Standards subcommittee for the Healthcare Surfaces Institute Surface Material and Product Certification program. Eastman, Solvay, and Roehm each had participants representing the thermoplastic industry, with Zebra Technologies representing original equipment manufacturers and PDI as the chemical disinfectant manufacturer representative.
Throughout 2020, participants met once a week to discuss current testing standards and to determine whether the standards could be more clearly defined, with the intent to facilitate ease of data comparison. Initially, each company provided feedback into their conditioning, exposure, and testing practices with accompanying justification. The companies chose testing methodology based on strong material science practice, relevancy to equipment manufacturer needs, end customer use cases, and reasonable material testing throughput.
All participating companies used or referenced ASTM D 543, ASTM D 638, and ASTM D 6110. Consensus was that the standards are defining testing, such as ASTM D638 Standard Test Methods of the Tensile Properties of Plastics and ASTM D 6110 – 04 Standard Test Method for Determining the Charpy Impact Resistance of Notched Specimens of Plastics, were sufficient in yielding comparable results from one experimenter to the next. However, ASTM D 543 left too much room for variation from experimenter to experimenter. Strain levels, exposure periods, and acceptance criteria were at the discretion of the experimenter and, as mentioned previously, produced a breadth of results, making data comparisons difficult.
The HSI Plastic Testing Standard Subcommittee proposed strains as a function of the material’s modulus. The new process grouped materials into strain brackets based on their moduli, mitigating the problem created when comparing material with significantly different moduli conditioned at the same strain levels. The minimum specimen amount was also set at five specimens per sample set within a “modulus bracket.” In addition to establishing strain brackets, two exposure periods were proposed: a short exposure period (24 hrs) and prolonged exposure period (7 days). By establishing exposure periods, like defining strain brackets, OEMs should experience reduced burden in data comparison when selecting materials for enclosures.
In addition to strain brackets and exposure periods, the HSI Plastic Testing Standard Subcommittee refined the test specimen exposure methodology. How chemical disinfectants are applied to test specimens varies from experimenter to experimenter. The HSI Plastic Testing Standard Subcommittee outlined guidance within the boundaries of ASTM D 543 for standard practices for applying chemical disinfectants to test specimens, removing another source of variance when testing thermoplastics of medical device enclosures. This bodywork was followed with proof-of-concept testing. The collaborative effort yielded a new integrated standard for conditioning, exposing, and testing thermoplastics. This integrated standard will hopefully provide harmony across the medical device design and manufacturing industry and guide plastic and chemical disinfectant manufacturers in their respective product development. As of March 2022, HSI filed a provisional patent for this test method as part of the HSI Surface Material and Product Certification program.
Future Objectives
The next immediate objectives are to open this subcommittee to include more participants. At this juncture, it is hoped that a professional body of scientists and engineers in the medical device space will champion this initiative and help drive it forward. Greater participation from the medical device design and manufacturing community would help this integrated standard move forward and evolve. An increase in participation and agreement will hopefully generate the necessary momentum for widespread adoption across the medical device industry, ultimately benefiting patient care through extended medical device enclosure design life.
This article was written by Michael F. Zettel, Senior Materials Scientist – Compatibility, PDI R&D, Woodcliff Lake, NJ; Yubiao Liu, Global Technical Lead in Medical Applications, Development Associate, Eastman Chemical Company, Kingsport, TN; Mark Lamont, Director, Mechanical Engineering, Zebra Technologies, Lincolnshire, IL; Kay Bernhard, Director of New Business Development – Medical, Roehm GmbH, Darmstadt, Germany; and James Hicks, Technical Development Engineer, Solvay Specialty Polymers, Alpharetta, GA. For more information on the subcommittee, contact Michael Zettel at



