With the shift in the medical industry to more minimally invasive, quicker, and more effective procedures, the goal is to minimize patient recovery times, reduce access incision sizes, and provide better patient outcomes through advanced medical procedures. This necessitates new medical devices that tend to be more demanding of their components than in devices past. This requires medical devices and their components to use advanced polymers. Many of these advanced materials fall under the general description of high heat polymers.
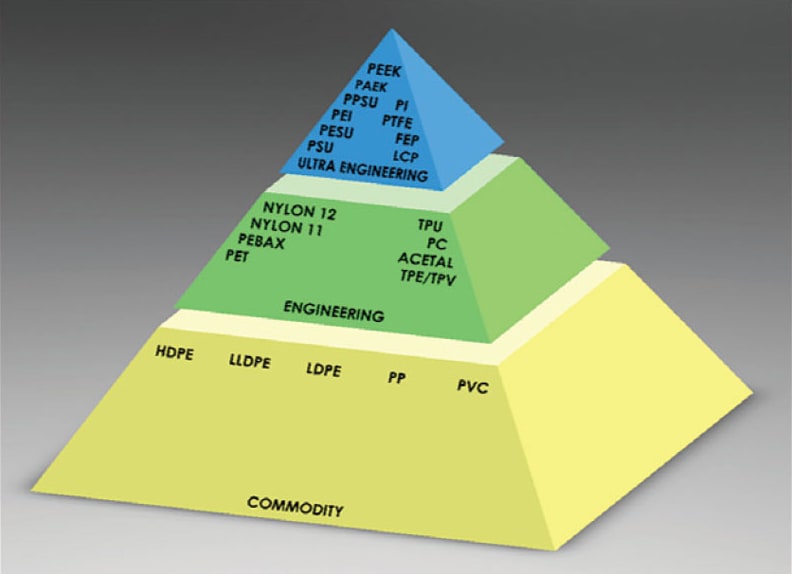
As noted in Part 1 of this article (January 2018), ultra-engineering polymers fall under the general classification of engineered polymers, yet they are at the pinnacle of performance for all thermoplastics. Ultra-engineering polymers bridge the performance gap between standard engineering polymers, such as nylon and polycarbonate; and metals, composite materials, and thermoset plastics like polyimide (see Figure 1). Their description of “high heat polymer” indicates not only that these materials are processed at higher temperatures, typically between 600° and 750° F+, but that they subsequently, also have high continuous operating temperatures, most well over 300° F.
Ultra-engineering polymers have very good chemical resistance, which makes them ideal for the hospital environment and the many harsh chemicals and drugs to which plastics can be exposed. The physical properties of ultra-engineering polymers also outperform all other standard engineered polymers in the areas of tensile strength, flexural strength, and impact resistance. Additionally, these materials have good dielectric properties and have some level of inherent flame resistance without additives. All the materials to be discussed in this article have USP Class VI and ISO 10993 approvals, and some have permanent implant-approved grades as well as MAF support.
All the materials listed here are suitable for the extrusion of profile shapes, multi-lumens, microbore tubing, large diameter tubing, thin wall tubing, rods, and filament. All can be compounded with additives, radiopacifiers, colors, and reinforcements but will have some limitations due to their high processing heats. Additionally, due to their high processing temperatures, tooling and processing equipment need to be specialized to withstand these high heats. Part 1 of this article focused on PEEK, PAEK, PEKK, FEP, and LCP. Part 2 focuses on amorphous polymers: polyphenylsulfone (PPSU), polysulfone (PSU), polyether sulfone (PESU), and polyetherimide (PEI), as well as some additional ultra-engineering polymers.
Amorphous Polymers
The remainder of the ultra-engineering polymers — PPSU, PSU, PESU and PEI — to be discussed are all amorphous and range from transparent to clear in appearance (Solvay Specialty Polymers 2016). An amorphous polymer does not have a distinct melting point and has no crystalline structure (see Figure 2).
PPSU

PPSU has the highest heat resistance of all the sulfones and PEI. PPSU has a continuous operating temperature of about 400° F, which allows it to handle some of the same high heat applications as PEEK. PPSU is very hydrolytically stable, which enables it to excel in high heat and humidity environments. PPSU is highly chemical resistant to all hospital solvents and wipe down chemicals (Solvay Specialty Polymers 2015) and also can withstand 100 or more cycles of all sterilization methods as well as 1000+ cycles of steam sterilization (Solvay Specialty Polymers 2015).
These properties, along with its transparency, allow this material to also bridge the gap between one-time use and durable devices where the ability to see the placement or position of catheters or endoscopy tools, or the flow of fluids is critical to the functionality of the device and procedure. PPSU has very high ductility, which is quantified by the lowest tensile and flexural strengths of all the materials discussed in this article. This high ductility positions this material as a candidate for steerable catheters and catheters that need to follow torturous paths through the body while still having need of high heat and chemical resistance. Permanent implant grades of PPSU are available for applications such as wire lead coatings, fluid transfer, and orthopedics (Solvay Specialty Polymers 2015).
PPSU is transparent, which means it can be colored with transparent tints to cover its natural transparent amber color. PPSU can be thermally formed for catheter applications as well as RF welded and reflowed, providing for easier assembly and connection methods for device and catheter assemblies. PPSU has the highest raw material costs of these amorphous materials, but it is still less expensive than PEEK. PPSU has a transparent amber appearance, which may not be aesthetically pleasing for some users. However, the amber color appears less pronounced in thin-wall and micro-bore configurations. This transparent amber color can limit how well it can be colored, especially with light colors, and may affect how vibrant bright colors may appear. The high processing temperatures of PPSU can also limit which colors and additives can be used because not all are thermally stable at those high heats.
PSU
The strength and toughness of the amorphous materials start to increase from this point forward. PSU is a high-strength sulfone material with greatly improved clarity over PPSU. PSU has the lowest continuous operating temperature of all these high heat polymers at about 340° F, but it is still considerably higher than other common engineered polymers. PSU has good chemical resistance to many hospital chemicals (Solvay Specialty Polymers 2015) and is hydrolytically stable for hot and humid environments (Solvay Specialty Polymers 2015). It can withstand 40 kGy of gamma sterilization and up to 100 cycles of all other sterilization methods (Solvay Specialty Polymers 2015).
PSU can be a choice for a durable component but it doesn’t have as long of a life span in some environments as the other materials covered in this article. PSU’s properties position it as a great, higher-end replacement for polycarbonate. PSU has better chemical resistance, higher hydrolytic stability, and much higher temperature resistance than polycarbonate (Solvay Specialty Polymers 2015).
Even though PSU can have a slight yellowish tint to it, PSU still has good clarity, which allows for a wider variety of colors and tints, while most grades of medical polycarbonate need to have a noticeable purplish tint to them to compensate for color shifts during gamma sterilization.
PSU has good ductility and better toughness. PSU is a good option for dental tools and components because of its toughness and hydrolytic stability. PSU comes in permanent implant grades that are not suitable for structural components but can be used in applications where ductility, strength, toughness, and clarity are necessary.
PSU can be thermally formed for catheter applications and can also be RF welded and reflowed, providing for easier assembly and connection methods for device and catheter assemblies. Raw material prices for PSU are moderate, about 50–75 percent higher than polycarbonate and similar to prices for PEI and PESU.
Some of the limitations of PSU include the following: it has decreased sterilization and chemical resistance compared with the other ultra-engineering polymers that are discussed here. And, because of its higher processing temperatures, some colors and additives can be limited because not all of those components are thermally stable at such high heats. PSU also has lower tensile and flexural properties than PESU and PEI but are higher than PPSU.
PESU
Another member of the sulfone family that has high potential for use in medical applications is PESU. PESU has the highest tensile and flexural strength of the sulfones discussed in this article. It also has excellent clarity. PESU has better chemical resistance to hospital solvents and disinfectants and has good hydrolytic stability (Solvay Specialty Polymers 2014). In combination with PESU’s continuous operating temperature of about 390° F, these properties make it a good candidate for harsh applications where high strength and clarity are needed for applications such as sight windows and patient access components where being able to see fluid movement, and device location and position are necessary.
Because of the stiffness and hardness of PESU, it has good pushability and torque properties that can potentially eliminate braiding and other reinforcement methods in some applications. PESU has just a slightly decreased physical and thermal properties compared with PEI, which makes it a good option for replacement of PEI while adding greatly improved clarity. PESU is also an option as a much higher performing alternative to polycarbonate with greater physical, thermal, and chemical properties and with only the slightest yellowish tint. But as stated earlier, polycarbonate needs to be tinted noticeably purplish to compensate for color shifts during gamma sterilization while PESU does not retain its clarity during all sterilization methods.
PESU can be thermally formed for catheter applications as well as RF welded and reflowed providing for easier assembly and connection methods for device and catheter assemblies. Raw material prices for PESU are moderate, and similar to prices for PEI and PSU.
PESU can withstand 4 megarads of gamma, greater than 1000 steam sterilization cycles, and 100 or more cycles of the other major sterilization methods (Solvay Specialty Polymers 2016). PESU is susceptible to environmental stress cracking due to exposure to certain families of solvents that can be present in the hospital environment (Solvay Specialty Polymers 2016). PESU has slightly lower tensile and flexural properties than PEI.
PEI

PEI is the final ultra-engineering material discussed in this article. PEI is commonly known by the brand name Ultem. PEI is the highest strength of the amorphous materials covered here, with higher tensile strength, flexural strength, and hardness than all of the sulfones. PEI’s continuous operating temperature is about 390° F (Saudi Basic Industries Corporation [SABIC] 2016) and is hydrolytically stable, as well as having better chemical resistance to many hospital solvents and disinfectants (Saudi Basic Industries Corporation [SABIC] 2014).
These properties allow PEI to be used in durable products such as device sheaths, access devices, and sterilization tray dividers and supports. Because of PEI’s strength and durability, it is also suitable for dental tool parts and fixtures. PEI can withstand greater than 1000 steam sterilization cycles, and it is suitable for gamma, ethylene oxide, and vaporized hydrogen peroxide sterilization processes (Saudi Basic Industries Corporation [SABIC] 2014). PEI also has excellent color stability through hundreds of sterilization cycles (Saudi Basic Industries Corporation [SABIC] 2014).
PEI can be thermally formed for catheter applications as well as RF welded and reflowed, providing for easier assembly and connection methods for device and catheter assemblies. Raw material prices for PEI are moderate, and similar to prices for PSU and PESU.
PEI has a transparent amber appearance, which may not be aesthetically pleasing for some users. This transparent amber color can limit how well it can be colored, especially with light colors, and may affect how vibrant bright colors may appear. PEI is attacked by some solvents that may be present in the hospital environment, and this attack can be exhibited by environmental stress cracking.
Other Ultra-Engineering Polymers
There are a variety of families of ultra-engineering polymers beyond those discussed in this article. These other high heat polymers tend to fall in similar property ranges as those defined by the materials discussed here. Most of these other polymer families have extrusion grades or grades suitable for extrusion.
These other materials are sometimes formulated for very specific application types or targeted for certain physical, chemical, or thermal properties and can subsequently have notable processing limitations that can dictate what types of parts and configurations are possible and the types of equipment that are necessary to process them. That is not to say that ultra-engineering polymers other than those listed here should be avoided by any means, because they can fill in and extend the performance gaps of the materials discussed here. Utilizing a processor with experience and knowledge of these other families of materials is key to the success of devices specifying other ultra-engineering polymers.
Metal Replacement
Despite all the high strength physical properties of the materials covered here, they still fall short of the performance of metals such as stainless steel. An ongoing goal for medical device designers and engineers is to find polymer solutions in applications that might typically use metals. Some of the benefits of polymer solutions to metals are lower product lifetime costs, more design and manufacturing flexibility, and a decrease of sterilization risks of multiuse devices.
Some of the challenges related to polymer substitutions for metals is still a relatively large performance gap between ultra-engineering polymers and metals. It is difficult to quantify actual physical needs of a project because of possible overengineering with stainless steel, and stainless is still such a legacy material that it can be difficult to present a polymer as a consideration.
One way that polymers can come very close to stainless steel performance is by adding fiber reinforcements into the polymer. Fiber reinforcement of ultra-engineering polymers increases all physical properties of the base polymer and can even improve thermal and chemical performance. Adding fiber reinforcement to a polymer drastically affects the polymer in multiple ways that need to be considered. The fibers can add unwanted properties to an extrusion because of the properties of the fiber, such as weight and conductivity. The extrusions can have increased stiffness but can seem fragile, especially in thin-wall and small diameter configurations. The fibers can affect surface finishes, but low fiber loadings of 10 percent or less can improve finishes while increasing properties and retaining ductility of the base polymer. Fiber loadings affect the processing and tooling design and require specially designed tooling to manage and compensate for fiber-reinforced ultra-engineering polymers.
Conclusion
Ultra-engineering polymers have added a level of performance that wasn’t possible until somewhat recently in the medical device industry. Navigating the properties and differences between ultra-engineering polymers requires those in development and specification roles to gain a new knowledge set.
This article has examined the amorphous polymers: PPSU, PSU, PESU, and PEI. Together with Part 1, which reviewed PEEK, PAEK, PEKK, FEP, and LCP, this article provides a high-level overview of these materials to help designers and engineers gain awareness of the many ultra-engineering polymers that are available for their extruded medical applications.
This article was written by Jonathan Jurgaitis, Senior Extrusion Engineer for Apollo Medical Extrusion Technologies (Sandy, UT). For more information, visit here .



