The use of bioabsorbable polymer materials in modern medicine is a major innovation. Known for their unique ability to safely exist in the body and eventually absorb without causing adverse interaction, bioabsorbable polymers have had a dramatic impact on device design and development over the past decade.
From sutures, staples, and fixation devices for surgical procedures, to stents for vascular and coronary applications, bioabsorbable material devices eliminate the need for a second invasive surgery to remove the device. This feature greatly reduces costs and the risk of complications, such as infection, associated with the removal procedure.
Molding Best Practices
Bioabsorbable polymers can be injection molded with extremely small features and thin sections. However, this process requires special dedicated molding equipment since many of these devices have small intricate 3D structures. The material can also be extremely sensitive to temperature.
“Temperature control is a critical factor,” says Norman Akens, molding manager at Medical Murray. “Absorbable polymer needs to be processed at the lowest temperature possible. If the material is exposed to elevated temperatures, this can quickly result in monomer formation and alter the mechanical properties.”
Due to both the sensitive nature and hefty cost of bioabsorbable polymers, it is essential to have dedicated processes and equipment in place that is developed specifically for injection molding small complex bioabsorbable parts. Using equipment that also produces parts from non-absorbable polymers introduces the risk of a non-absorbable material being introduced to a supposedly absorbable component.
The company designed, patented, and uses a molding machine called the Sesame™, to provide fast controlled injection and short material residence time. It uses a pneumatically driven vertical plunger for plasticating, and injects the material with a tiny horizontal plunger (1.0 to 2.5 mm diameter) driven by a linear servo motor. Total injection time can be as short as 0.02 seconds.
The molding machine’s features include very low melted volume, controlled high speed, and high pressure injection. This allows for the molding of sub-micro size parts with complex geometric features from materials including bioabsorbable polymers that would otherwise degrade in standard molding equipment.
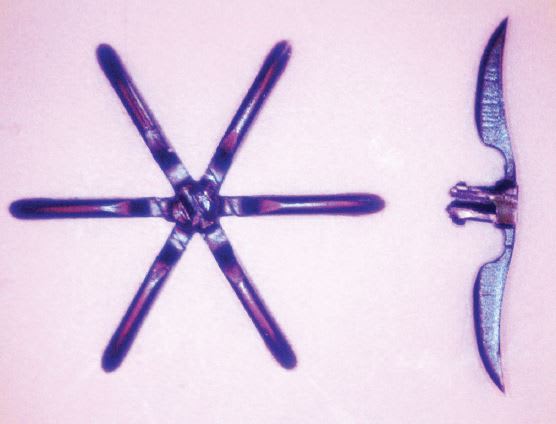
In addition, the company was instrumental in developing processes and designs to create miniature hinges in molded devices for bioabsorbable fasteners that are activated during placement. The Sesame machine has been used to produce parts that measure just 0.10 mm and weigh only 2.5 mg, or 1/25th of a single pellet. (See Figure 1)
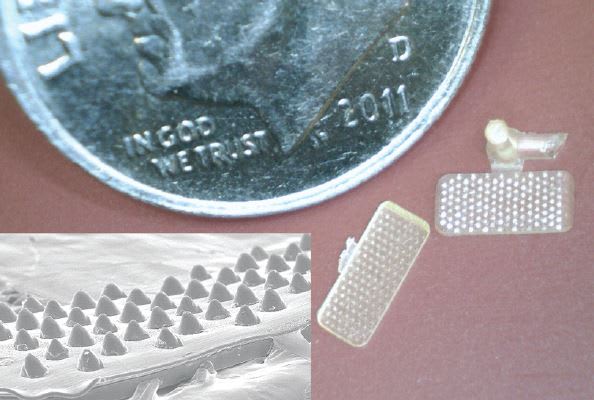
Conventional screw plasticizing machines commonly require sprue and runner systems that consume 10 to more than 100 times of the volume needed to produce one part. Medical grades of bioabsorbable resins can sell for upwards of $13,000 per kilogram. Therefore, scrap material must be tightly controlled to minimize the effect on a company’s bottom line.
Figure 2 features a bioabsorbable grip with runner and sprue attached. The inset image at bottom left shows the part magnified using an electron microscope with each individual grip 138 microns high and 123 microns base diameter. The overall part size is .19" L × .08" W. Given the high cost of bioabsorbable materials, minimizing the total shot size is critical to maintain overall cost effectiveness.
“The customized equipment that Medical Murray utilizes allows scrap material to be reduced to as low as two percent, reducing costs significantly for small absorbable injection molded components,” Akens says.
Other Performance Criteria
To achieve success with bioabsorbable materials, it is essential to use consistent materials throughout the development and manufacturing process. Careful storage and monitoring of materials prior to and after the molding process is critical. To extend bioabsorbable polymer material shelf life, material should be dry and shielded from any possible moisture.
Material testing should also take place, including:
- Flow Data,
- Molecular Weight, Inherent Viscosity
- Moisture Content,
- Tensile Strength, Elongation, and
- Surface Hardness.
Conclusion
There continues to be excitement around the capabilities of bioabsorbable polymer materials. Whether they are used to facilitate a controlled drug delivery function for stents and grafts or engineered for wound closure, the possibilities are endless.
When deciding to use bioabsorbable materials in your next project, it is essential to use a dedicated engineering team that fully understands the temperamental nature of this material and the cost parameters. A careful combination of the correct mold design, correct machining processes, and consistent material from start to finish will ensure continuous design success.
This article was written by Tanner Hargens, Regional Vice President for Medical Murray’s Southeastern division located in Charlotte, NC. The company is based in North Barrington, IL. For more information, Click Here



