
The global medical tubing market is enjoying strong growth, with analysts forecasting compound annual growth rates (CAGR) ranging from 6 to 9 percent over the next several years. Demand is being propelled by more-frequent or extended treatments for cancers and chronic diseases; increases in minimally or noninvasive surgery, home-based care, and patient wearables; and innovations such as sensor-enabled smart catheters and other advanced tubing applications.
However, tubing manufacturers also face potential headwinds. In the area of materials, anticipated regulatory restrictions on per- and polyfluoroalkyl substances (PFAS) and rising costs are prompting the industry to explore alternatives to fluoropolymers, a category of PFAS chemicals.
Two promising options are polyetherimide (PEI) resins and PEI-siloxane copolymers. These specialty thermoplastic materials not only are formulated without fluorine but also deliver desirable properties such as high heat and chemical resistance and low friction. They also provide easier and faster processing than fluoropolymers, contributing to higher productivity and potentially lower system costs.
Before considering PEI resins and PEI-Si copolymers for medical tubing applications, manufacturers first want to ensure they meet baseline requirements: biocompatibility, particularly hemocompatibility for use with blood; and the ability to be sterilized using methods such as ethylene oxide (EtO) and gamma radiation.
SABIC’s ULTEM™ PEI resins and SILTEM™ PEI-Si copolymers are supported by a comprehensive healthcare product policy whose requirements specify biocompatibility testing (e.g., ISO 10993 or USP Class VI), maintaining stringent formula lock and change management processes, and providing access to U.S. Food & Drug Administration (FDA) Master Files. These products are designated by HU family grade names.
These materials are compatible with many commonly used sterilization methods, including gamma. For EtO sterilization, testing of the SABIC materials is currently under way and is expected to demonstrate compatibility.
Because of these essential attributes and other desirable properties, PEI resins and PEI-Si copolymers can potentially replace fluoropolymers in medical tubing used in catheters, cannulas, and other applications on a case-by-case basis, helping manufacturers address regulatory and processing challenges.

Fluoropolymers Under Regulatory Scrutiny
Fluoropolymers such as polytetrafluoroethylene (PTFE), fluorinated ethylene propylene (FEP), and perfluoroalkoxy (PFA) have long been regarded as the gold standard for medical tubing due to their combination of low friction and resistance to chemicals and high heat. However, because these high-molecular-weight polymers with fluorine atoms in their chemical structure are a category of PFAS, their use — and possible restriction — in medical applications are being debated.
In the United States, the FDA recently concluded in an August 2025 article titled “PFAS in Medical Devices” that “currently there is no reason to restrict their continued use in devices.”1 On the other hand, the European Union is considering a sweeping PFAS ban under the REACH framework, and individual member states such as Denmark have imposed their own restrictions. In Asia, countries including China, Japan, South Korea, Australia, and New Zealand have prohibited or restricted PFAS substances.
Given the variable and evolving PFAS regulatory landscape, medical tubing manufacturers may be considering transitioning to fluoropolymer alternatives.
Replacing fluoropolymers in tubing applications across the board is not considered feasible at the present time, as no single material can serve as a substitute in every case. But nonfluorinated PEI resins and PEI-Si copolymers can replace fluoropolymers in certain applications to help address regulatory concerns about PFAS. And compliance is not the only advantage these materials offer.
Benefits Beyond Regulatory Compliance
Polyetherimide resins are amorphous thermoplastics known for their outstanding thermal resistance (glass transition temperature of 217 °C), high strength, and broad chemical resistance. They are inherently flame resistant without the use of halogens or other additives. Polyetherimide-siloxane copolymers provide similar characteristics but also offer improved tensile elongation (flexibility) versus PEI resins, due to incorporation of siloxane soft blocks.
Compared to fluoropolymers widely used in medical tubing (i.e., PTFE, FEP, PFA), PEI resins and PEI-Si copolymers deliver similar transparency, colorability, flexural and storage modulus, and tensile elongation at break. Further, in applications such as tubing liners, the lubricity performance of PEI materials is typically adequate to meet requirements. If enhanced nonstick capability is needed, lubricated PEI resins are currently available and lubricated PEI-Si compounds are under development. All these attributes make PEI materials suitable for select tubing applications.
While fluoropolymers — particularly PFA — exhibit excellent chemical resistance due to their strong carbon-fluorine bond, inertness, and thermal stability, PEI resins and PEI-Si copolymers also withstand degradation from harsh chemicals, including those used in healthcare settings.
Heat resistance is another feature of PEI-based materials. Their high continuous service temperatures enable processing and sterilization performance similar to those of fluoropolymers, which may be overengineered in high-temperature resistance for some applications.
Regarding sterilization, as mentioned above, PEI materials offer broad compatibility with popular methods (steam autoclave, vapor hydrogen peroxide, ultraviolet-C, etc.) and can provide better resistance to gamma radiation than fluoropolymers. Testing has shown that PEI resins and PEI-Si copolymers resist color shifting and retain key properties such as tensile elongation and multi-axis impact resistance after hundreds of sterilization cycles.
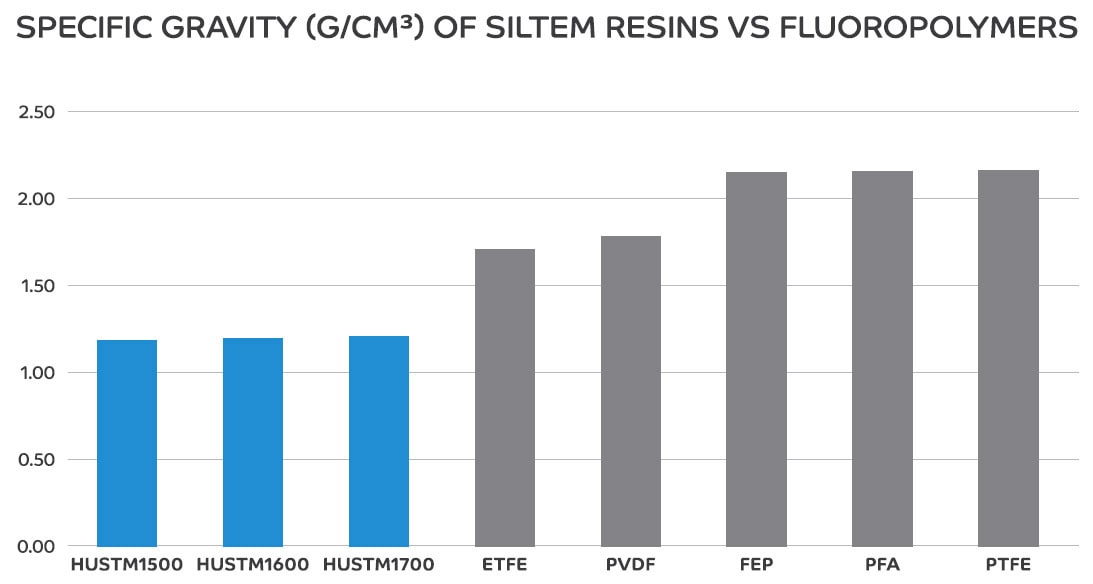
Further, PEI resins and copolymers have lower specific gravities than fluoropolymers, as outlined in Figure 1, which can reduce part weight and raw material costs.
There are also distinct differences in processability between fluoropolymers and PEI-based materials. The former are characterized by tight processing windows, melt fracture sensitivity at high shear rates, and the potential to release corrosive or toxic byproducts under exposure to very high temperatures.
The latter deliver these processing advantages over fluoropolymers:
Corrosivity avoidance, so they can run on conventional equipment.
Faster line speeds (up to 600 m/min.) with lower sensitivity to melt fracture and better surface finish.
Lower draw-down ratios.
Wider processing window.
Easier and faster processing can help manufacturers meet expanding market demands for medical tubing.
Opportunities to Replace Fluoropolymers
Currently, PTFE tubing is commonly used for catheter liners and sheaths. As an alternative to PTFE, extruded FEP tubing can be used in catheters and other medical devices — typically for low-pressure microfluidic applications. Also, FEP heat-shrink tubing is employed as a manufacturing aid to bond and fuse components of catheters.
Polyetherimide resins and PEI-Si copolymers can potentially serve as alternatives to specific fluoropolymers in applications such as liners for small-diameter, thin-wall tubing and cannulas for insulin pumps.
Conclusion
Polyetherimide resins have a long history of success in medical device applications such as surgical instruments and trays, equipment housings, and drug-delivery components. They meet stringent standards for biocompatibility and are compatible with multiple sterilization methods. Looking ahead, PEI-Si resin blends may also enjoy similar success in the healthcare sector.
Today, in certain tubing applications, both PEI resins and PEI-Si copolymers can replace fluoropolymers to enable PFAS regulatory compliance and enhance processability. Compared to other potential replacement materials such as PEEK, PEI materials are more cost-effective and flexible. Overall, these specialty resins and blends provide biocompatibility, broad sterilization capability and regulatory compliance, paired with high performance and efficient processing — an attractive combination for the industry.
Reference
- “PFAS in Medical Devices,” U.S. Food & Drug Administration, current as of 08/06/2025.
This article was written by Sean Culligan, Senior Manager, ULTEM™ and NORYL™ Resins; Manish Nandi, Business Development, Healthcare; and Nithin Raikar, Sr. Business Manager, Global Product Management at SABIC. For more information, visit here .



