
The global medical device market is projected to reach a value of $656 billion USD by 2032 with a CAGR of 3 percent over the coming decade.1 The preceding decades of globalization and increased prosperity has provided advancement in both medical technology and access to advanced medical care for a greater proportion of the world’s population. Further, an aging population in North America, Europe, and parts of Asia will increase the need for healthcare-related services and medical devices in the coming decades. At present, the North America market continues to dominate the industry, accounting for approximately 43 percent of the market’s revenue share; however, markets in the Asia-Pacific region have the highest expected growth rates in the coming decades.1 Growth and innovation in the medical device market will be critical in the years to come.
The adhesive bonding of plastics used in medical devices will be the primary focus of Part 1 of this article. Part 2, which will appear in a future issue, will cover the material properties of plastics. Solvent bonding or ultrasonic welding may also be used for forming bonds, but their use is limited when joining dissimilar materials. Ultrasonic welding of dissimilar plastics requires similar melting points, thermal expansion characteristics, and a degree of compatibility between the differing polymers in question. Due to their wide range of material and chemical properties, plastics are amongst the most challenging substrates to bond with adhesives. Considerations such as surface energy, degree of crystallinity, coefficient of thermal expansion, and elasticity pose particular challenges.
A general understanding of the material properties of plastics as well as understanding the theories of adhesion are beneficial to the design process. Despite these considerations, the wide formulation latitude possible with adhesives as well as their ease of use in a manufacturing environment make them a cornerstone of medical device construction. Factors and requirements of particular note for medical devices include biocompatibility/cytotoxicity, sterilization resistance, reusable versus disposable devices, temperature and chemical resistance, coefficient of thermal expansion, surface preparation of plastics, and the general material and rheological properties of the adhesive. Understanding the different theories of adhesion also provides insights to the design and troubleshooting process when using adhesives.
Factors and Requirements
In the United States, medical devices are regulated by the Food and Drug Administration (FDA) and, generally, are assessed under USP Class VI biocompatibility standards. The degree of regulatory requirement for a particular device is based on risk; the risk as well as the stringency of the requirements increases from Class I through Class III. Medical devices encompass a broad range of products from endoscopes and catheters through complex, life-critical devices such as ventilators and pacemakers.
The design and construction of medical devices encompasses a wide range of materials including metals, glass, ceramics, micro-electronic components, and, frequently, a variety of different plastics. Joining and bonding dissimilar materials poses an engineering challenge that is frequently met by specially formulated adhesives. To ensure patient safety, medical devices as well as the adhesive they employ must meet rigorous safety requirements for biocompatibility and cytotoxicity such as U.S. Pharmacopeia Class VI biocompatibility and/or the ISO 10993-5 cytotoxicity requirements.
Biocompatibility. A finished medical device must comply with biocompatibility standards such as USP Class VI or ISO 10993-5. The nature of this regulatory landscape is complex and will depend greatly upon the nature of the medical device in question and the level of risk it imparts to the patient. The methodology of in vivo or in vitro testing may be used in assessing cytotoxicity and biocompatibility. Extraction solvents such as polyethylene glycol or a sodium chloride solution may be used to leach out any migratory or deleterious components that may be present in the material to be tested.3
In the case of in vitro testing, these leachates may be introduced to cell cultures and monitored for signs of cytotoxicity.4 In the case of in vivo testing, leachates may be injected intravenously or intracutaneously into a laboratory animal with subsequent monitoring and assessment of negative effects. Systemic toxicity may also be assessed by full implantation of a medical device into a laboratory animal. A detailed discussion of the biocompatibility and cytotoxicity requirements are beyond the scope of this article as they are highly specific to a particular type of medical device, its intended use, and its destination market.
Sterilization. Resistance to sterilization is a significant factor when designing a medical device. Sterilization may be accomplished by a variety of means including high-temperature steam autoclaving, gamma irradiation, or chemical sterilization using agents such as ethylene oxide (EtO), hydrogen peroxide, or other reactive chemicals. The life cycle of the device, whether disposable or reusable, as well as the level of risk posed by the device will impact the design and engineering considerations with respect to sterilization.
High-temperature sterilization poses particular engineering challenges due to the pressurized humid environment and differential thermal expansion between the materials of construction.2 High-pressure water vapor may be absorbed into the adhesive, compromising modulus or other material properties; further, incursion of moisture at the interface may result in adhesion loss and resulting bond failure. It is important that an adhesive used for high-temperature steam autoclaving be temperature and moisture resistant with a high glass transition temperature (Tg).
Further, in the case of chemical sterilization methods, the adhesive must have a suitable degree of chemical resistance to the sterilization agent. Inherently, chemical sterilization agents are highly reactive; generally, promoting chemical cross-linking or acting as strong oxidizers. Selection of an appropriate adhesive requires assessing its degree of chemical resistance; polymer systems like epoxies offer strong chemical resistance, temperature resistance, and a high modulus. Epoxy adhesives are generally rigid and possess a high degree of strength; in applications where a high degree of flexibility is needed, silicone adhesives provide an alternative solution.
The flexibility of silicone adhesives aids the dissipation of accumulated stresses that result from differential thermal expansion of dissimilar materials within a joint. There is a degree of interplay between temperature and chemical resistance. At high temperatures, the increased kinetics of deleterious reactions between a cured thermoset adhesive, sealant or coating and a chemical increase the risk of chemical attack and degradation. Further, at high temperature, thermoset polymers will generally soften increasing the ability of reactive agents to diffuse within the polymer network and degrade the physical properties of the polymer. Epoxy systems with a high glass transition temperature (Tg) and high hardness values will tend to resist softening at high temperatures thus limiting the diffusion of reactive agents into the bulk of the adhesive. The extent of cross-link density will also limit diffusion, increase intrabulk cohesion, and contribute to both high temperature resistance and chemical resistance.
Disposable vs. Reusable Devices
Generally, the product hierarchy of medical devices can be separated into disposable and reusable devices. This distinction is important when determining the extent of resistance that the adhesive and the resulting medical device must face. In the case of disposable devices, they generally only undergo one sterilization cycle — often bulk sterilization using EtO or gamma irradiation during their manufacturing process — and thus face less rigorous demands when compared with higher-value, reusable devices that may undergo many repeated sterilization cycles.
With regard to practical concerns such as manufacturing throughput, disposable medical devices are generally high-volume manufactured articles that require a maximum degree of production efficiency to maintain appropriate margins. Here, rapid assembly is key; high-speed automation of adhesive dispensing, rapid fixturing, and rapid cure are critical to achieving profitability and economies of scale. Therefore, UV or LED light-curable adhesive systems or those that employ a dual-cure mechanism offer significant advantages. Compared with thermal cure mechanisms, UV or LED-curable adhesive systems can be cured instantaneously upon irradiation with a high intensity UV or LED light source.
Dual-cure systems may use a quick exposure to a UV or LED light source to assist with fixturing while then exposing the part to high temperature post-cure somewhere in the downstream manufacturing process. Depending on the photoinitiator used in a UV curable system, it may be possible to cure the adhesive through a UV or LED-transparent material, i.e., to instantaneously cure the adhesive within the bond-line itself. Since many clear, amorphous plastics have a degree of UV or LED transparency, this method provides particular advantages within a manufacturing process. In the case of reusable medical devices, adhesive requirements will most heavily focus on resistance to aggressive and repeated sterilization. The higher-value and longer-service life of these devices make production speeds less sensitive.
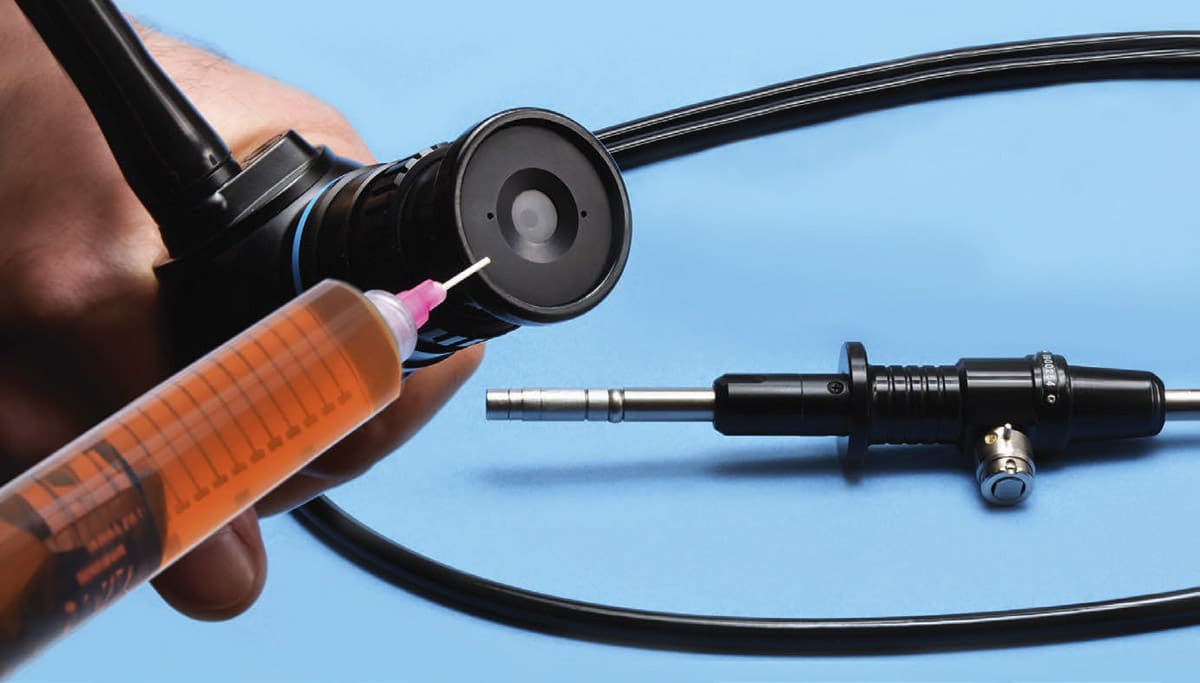
The coefficient of thermal expansion (CTE) varies by material; upon heating and cooling, differential expansion between different materials of construction can lead to the accumulation of stresses at the bond-line that may result in adhesion failure. Adhesives can be formulated to mitigate the risk of adhesion failure resulting from differential thermal expansion. This is of particular concern with devices such as endoscopes.2 In endoscopes, various optical glasses have different CTE values and may be glued together to form an assembly; during the autoclave cycle of heating and cooling, the adhesive must be able to not only handle the high heat and steam, but also withstand the stresses that accumulate due to differential thermal expansion.
Polymeric materials used in adhesives often have CTE values that are much larger than inorganic materials such as glass and metal; often, the CTE of polymeric materials may be an order of magnitude greater or more. To address this, adhesive formulators can use functional inorganic fillers to tailor the CTE of the resulting adhesive to meet the demands of the product and its operating requirements.
Conclusion
Part 1 of this article has examined adhesive bonding of plastics in medical devices. The set of desired properties for medical-grade adhesives ultimately depends upon the specifics of the application and the kind of sterilization process that will be used. Exceptional resistance to high temperatures and moisture are a must for adhesives subject to repeated autoclaving, while outstanding resistance to chemicals is required for adhesives that will face chemical sterilants. Part 2 will cover the material properties of plastics and how key properties of plastics relate to adhesive bonding.
Reference
- Market.Us, “Medical Devices Market Size Globally", Yahoo Finance, 2023. Accessed: July 28, 2023.
- Ballhorn, Michael, “Bonding in Medical Technology,” MEDengineering, 2013. Accessed: July 28, 2023.
- Wallin, R., “A Practical Guide to ISO 10993-5 Cytotoxicity,” MD&DI, April 1998. Accessed: July 28, 2023.
- “USP Class VI Testing,” SaniSure. Accessed: July 28, 2023.
This article was written by Venkat Nandivada, Manager of Technical Support, and Rohit Ramnath, Senior Product Engineer, at Master Bond, Hackensack, NJ. For more information, visit here .



