Silicone materials have been around for more than 70 years and since the 1960s have played an important and evolving part in products designed for the medical field. Since that time, silicone materials have been developed in various medical grades for skin contact, as well as short-term and long-term implantation. These materials can be formed into products using compression molding, transfer molding, and injection molding. This provides engineers, designers, and inventors with a high level of flexibility when selecting a molding process to produce their medical devices. Medical devices such as tubing for medical diagnostic systems to drug delivery devices like tear duct (punctual) plugs have been produced out of silicone.
History
The first commercially viable silicone product was created by Dr. J. Franklin Hyde of Corning Glass. This product was used to impregnate and coat glass cloth for electrical insulation purposes. In 1943, Corning Glass and The Dow Chemical Company formed the Dow Corning Corporation to pursue the commercialization of silicone materials. In the 1950s, High Temperature Vulcanization (HTV) and Room Temperature Vulcanization (RTV) materials were added to many silicone suppliers’ product lines. While these materials are still used today, they are not as common as Liquid Silicone Rubber (LSR) or High Consistency Rubber (HCR).
HCR, a form of silicone typically referred to as gum stock, was introduced in the late 1950s to early 1960s by various silicone suppliers. HCR was most suitable for transfer molding, which is today, a legacy technology. LSR was introduced in the 1970s and today is the most common material used in molding medical device components. LSR provides medical device manufacturers a hypoallergenic material with a high level of biocompatibility, a high degree of thermal stability, and extreme temperature range performance, while being resistant to bacteria and mold growth. As time has progressed, silicones have been made available that are refined further for medical device use beyond skin contact. These newer cutting edge materials can be used inside the body for short- and long-term use.
Materials
Medical device designers, engineers, and inventors have many silicone material choices available today. Many medical grade silicone materials have been formulated by silicone manufacturers to meet the demands and requirements of various medical devices. Silicone suppliers have created guidance for the use of these materials by creating in-house certifications for bio-contact applications. In order for these materials to become certified, they must pass a series of United States Pharmacopeia (USP) tests. The USP creates product quality guideline standards for regulatory agencies and manufacturers of medicine, food ingredients, and medical devices.

The silicone materials currently available to medical device manufacturers include medical non-implantable, medical restricted implantable, and medical unrestricted implantable. The selection of each material is dependent on the medical device’s end use. The higher USP grade of material directly correlates to the purity of the material and level of expected response by the body.
Medical non-implantable materials are widely available today. Many of the silicone suppliers manufacture this grade of material in a variety of durometers so that medical devices of different hardness can be made. Non-implantable medical grade materials are acceptable for skin contact. However, they cannot be used inside the body. Many food grade materials are also in this category. That is the main limitation of this grade of material.
The next step up in medical grade silicones is the restricted grade for implantation. Materials belonging to this group are typically only able to be implanted for 29 days or less. These materials are used for implants that are needed for short-term solutions, often related to surgical procedures. In addition, these materials can be loaded with a low percentage of barium or tungsten to be detected inside the body using X-rays, CT scans, and even magnetic resonance imaging.
The most advanced and expensive medical silicone grade available is the unrestricted grade for implantation. These materials can be implanted into the body for beyond 29 days. Essentially, this form of silicone used is for long-term implants that may remain in the body until end of life. Materials in this group, will undergo all USP tests as they are refined the most and inside the body for long periods of time. As with restricted grades of silicone material, these materials can be loaded with a low percentage of barium or tungsten for future medical detection inside the body.
There are many medical grades of silicone available from the premier silicone suppliers today. The three grades of medical silicone materials can be accessed by designers, inventors, and engineers with relative ease. If a certain project requires a material with special additives and or characteristics, then many of the silicone material suppliers will work with your company to create custom batches. The availability of medical grade silicone is better than ever and datasheets can be accessed via the internet.
Mold Processing
Silicone materials can be formed into parts utilizing multiple molding processes. In order to determine which process is used to mold the parts, many manufacturing variables must be reviewed. Four variables that immediately come to mind are: phase of the product lifecycle, production volume, part geometry, and material type.
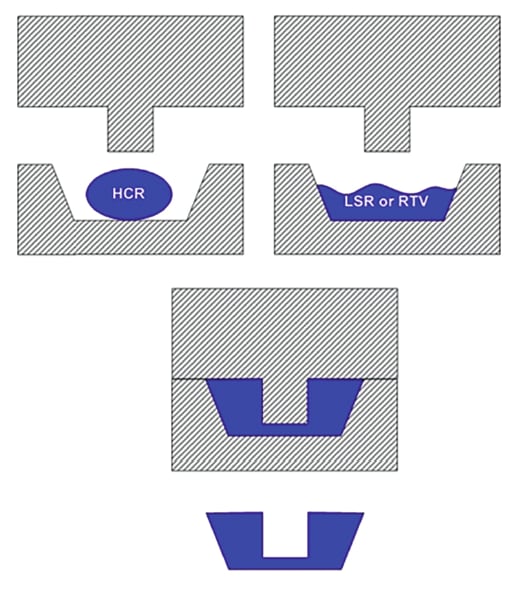
Depending on where the part design is in the product lifecycle will largely determine which molding process is used. Compression molding offers a flexible option when the part design is in the prototyping, testing, and product launch phases. Compression mold tools can be altered in design and modified easily as design changes are made. Once the part design is finalized, then testing can be completed. As the medical device outfit prepares its silicone part for product launch, compression molding still offers a viable option. Compression molding allows for an easy switch between different material grades and durometers for testing purposes, while providing a dimensionally correct product in the right material. (See Figure 1)
The part geometry will become a major factor as to which molding process can be used. If the part is simple in design and does not require undercuts or complex cores, then it can be injection molded or compression molded. However, if the part is highly complex in geometry and requires multiple cores then compression molding may be a better fit. It is important to note that complex tooling can require very extensive investment in injection molding tools as compared to compression.
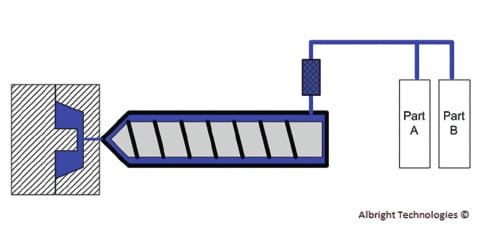
The final variable that will determine what form of molding is selected is the material type. LSR is suitable for compression and injection molding. This is the most common and advanced silicone material widely available today. LSR is inserted into compression molds via dispensing guns, while it is fed to injection molds via meter and mixing systems. HCR utilizes a process known as transfer molding to mold silicone products. Transfer molding rams HCR into a mold tool and then transfers it into the cavities. Since HCR is a legacy material, its use is declining, while LSR’s usage continues to increase. The reason for this is that the properties that once made HCR desirable can now be achieved using LSR.
Applications

Silicone has seen an increased utilization rate since the 1990s in the medical industry. As medical devices become more complex and demanding, silicone offers designers an excellent material to work with in terms of flexibility and stability. The material is capable of handling a diverse temperature range, with great tear strength and elongation properties. Silicone molding offers medical device companies and start-ups a viable solution for their most demanding products.
Silicone is commonly used in surgical tools for many reasons. Metal tools are commonly overmolded with silicone to form handles. In addition, seals are typically made of silicone within these surgical devices to reduce potential leak paths. Guards on surgical tools are made of silicone to protect surgeons and patients from sharp edges, insulate electrical currents, and dissipate heat. Many contact seals for instrumentation used to insert medical device implants in the body are also made of silicone. (See Figure 3)
Many delivery devices used inside hospitals and lab testing facilities are commonly made out of silicone. Tubing used in drug delivery, such as peristaltic pumps, and surgical procedures is formed of silicone. Face masks used in sleep apnea and other breathing applications are molded from silicone as well. Low reactivity and low immune response makes silicones good candidates for handling critical medical and pharmaceutical applications.
Keypad covers used in medical diagnostic machines are made out of silicone and often coated with parylene coatings. Another growing use of silicone is the encapsulation of electronics used in certain medical devices. This provides advantages as the electronics are insulated and the heat that is created can be dissipated through the layer of silicone and made waterproof.
Conclusion
As medical silicone materials continue to evolve and have their characteristics enhanced by suppliers, medical device companies will continue to increase their adoption of these materials. Medical devices will continue to shrink in size, become more complex and more intricate in the 21st century. Silicone will provide medical device designers and engineers with a proven and flexible material to assist in the creation of their new products. Medical grades of silicone will become even more valuable to the medical device field with each passing year, as additives and other enhancements are introduced to fulfill further product demands.
This article was written by Ryan M. Taylor, Business Development Specialist, Albright Technologies, Inc., Leominster, MA. For more information, Click Here . BIOMEDevice, Booth 700; MD&M East, Booth 2223



