Many of today’s medical applications use high-quality silica optical fiber. Because a broad range of optical fibers is available to serve this market, users must carefully choose the right fiber to avoid delays in product design and time to market, along with increased development costs.
The fiber core typically consists of silica doped with germanium to increase the refractive index. The fiber cladding is made of either pure silica or polymer material and features a slightly lower refractive index than the core to help achieve total internal reflection. Users may custom order the refractive index of the core and/or cladding to meet their specific needs.
The outermost fiber layer, called the coating or buffer, provides mechanical protection for the fiber. In many medical applications, manufacturers may use biocompatible coatings such as acrylate, ethylene tetrafluoroethylene (ETFE), polyimide, or silicone. They may also apply additional jacketing materials including polyurethane, Pebax®, or medical grade PVC to provide extra protection.
Characteristics of Optical Fibers
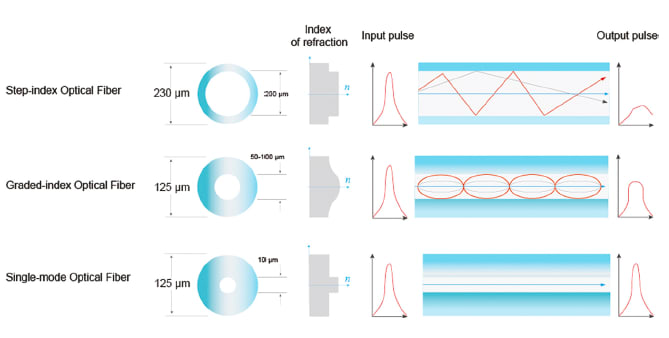
Single-mode optical fibers feature a small core size (typically less than 10 μm) and transmit signals using only one mode in the fiber core without modal dispersion. Step-index multimode fibers have a larger core (from 40μm to 1500μm) with a flat refractive index profile along the core’s radial direction which produces modal dispersion. Graded-index fibers have a smaller core size (usually 50 μm, 62.5 μm or 100 μm) with the refractive index profile gradually reducing from the center of the core out along the core’s radial direction (See Figure 1). Customers often first consider these structural and optical characteristics when selecting a fiber.
Single-mode fibers are most often used for communication applications because of their excellent signal delivery capabilities. In the same way, this fiber type is now increasingly used in medical applications, such as in optical coherence tomography (OCT) catheters to deliver image signals, or as an intrinsic sensor with fiber Bragg gratings (FBG). Developers are creating numerous medical uses for single-mode fibers because many of these fibers offer a broad range of operating wavelengths.
Users often choose polyimide-coated single-mode fiber for applications where a small biocompatible fiber is needed such as inside of a guidewire or a catheter. In many medical procedures, the catheter or guidewire is subjected to extensive bending, twisting, and spinning. In these cases, bend insensitive fibers provide better signal quality.
Multimode Fibers
Almost all laser surgeries involving optical fiber use step-index multimode fibers. Factors such as the required minimum bend, robustness, and ablation area size are valuable in making the right fiber choice.
The size of an optical fiber dictates its minimum bend radius (MBR). However, even when the manufacturer’s MBR recommendations are followed, light leakage can occur. This leakage usually occurs when high peak pulse energy, transmitted through tight bends, damages the fiber coating and leads to a fiber break during the procedure. For example, leakage can occur during lower pole kidney stone ablation.
To avoid this problem, designers developed an optical fiber with glass core/cladding and polymer coating to provide improved reliability and better bend performance during laser transmission.
Fibers range downward in core size from step-index fibers to graded-index fibers and then on to single-mode fibers. Aa single-mode fiber core is approximately 10 times smaller than a graded-index fiber core. Graded-index fiber cores are often used for interferometry-based sensing applications such as pressure or force sensing. A key reason for this choice is that the signal distortion from a graded-index multimode fiber is lower than that emanating from a step-index multimode fiber. In addition, the graded-index profile can be used as a lens to expand or collect the light beam based on direction. In OCT, graded-index fiber is typically used with single-mode fiber to comprise a signal probe (coreless fiber is typically used together as a spacer).
Multicore Fiber
Multicore fiber earned its name from a differing structure that features multiple fiber cores. Multicore fiber can be designed with different cores inside of cladding to serve specific applications. It is important to note that designing and producing multicore fiber can be much more expensive than stacking different fibers together. Multicore fiber is most typically used when form factor is a concern. Many projects are underway to design and develop multicore fiber for use in sensing applications.
In medical applications, multicore fiber is used as a shape sensing fiber. To construct this fiber, three single-mode cores are positioned into a triad shape (or six cores to make two triads) and the cores are then twisted along the entire length of the fiber during production. The shape of the fiber can be reconstructed on a display monitor by calculating each core’s bend or twist stress. Researchers are studying the use of shape sensing technology in minimally invasive surgery and diagnostics such as needle biopsy.
Coreless Fiber
As the name indicates, this fiber type lacks a core. With this structure, the light beam dissipates through the buffer instead of being guided through a core. Coreless fiber is often used as a termination fiber at the end of other fibers. In medical applications, this fiber is typically used as a beam buffer or a spacer for beamshaping applications as mentioned above.
Microstructure Optical Fiber
Microstructure optical fiber is often referred to as “holey fiber” or photonic crystal fiber (PCF). While traditional optical fiber uses the refractive index difference between the core and cladding to guide light, this fiber uses the shape of the microstructure to confine the waveguide within the core. While microstructure fiber is not yet commercialized, it offers very distinct advantages and characteristics.

Best Practices
Several companies design and manufacture silica optical fibers. To help avoid setbacks during and after FDA 510(k) approval, here are some key points to consider when evaluating a medical sub-assembly manufacturer:
- Verify that the supplier understands the medical industry and can provide quality support along with ISO 13485 certification.
- Confirm that the supplier follows FDA good manufacturing practices.
- Ascertain that the supplier maintains Device History Records and retains data for an established period of time.
- Inquire about the availability of Environmentally Controlled Manufacturing Rooms for low bioburden assembly manufacturing. (See Figure 3)
- Confirm that the supplier uses a vertically-integrated manufacturing system: preform, fiber, cable, and assembly.
- Verify that the supplier maintains biocompatibility test records and certificates.
- Ask whether the supplier employs proven validation and quality control processes such as: design and process validation plans; IQ, OQ, PQ; manufacturing control systems, etc.
- Confirm that the supplier has experience supporting new product development, and has the capability to scale up to volume production.
This article was written by Jaehan Kim, Medical Market Manager, and Jonathan Loft, Medical Account Sales Manager, OFS, Avon, CT. For more information, Click Here .



