The analytical techniques currently available to monitor chemical and biochemical production processes are difficult to apply in real time. Recent advances in solid-state fiber optic sensing technology allow new techniques to complement the existing tool kit. The use of optical in-line sensing enables real-time monitoring of production status, highlighting problems as and when they occur.

Process Analytical Technology
The U.S. Food and Drug Administration’s (FDA) Process Analytical Technology (PAT) guidelines provide an idea of what can be achieved in improved production processes based on such real-time analysis. This search for real-time information, offering increased efficiency and improved process control, echoes the FDA’s drug quality guidelines. The guidelines adopt the Quality by Design (QbD) principles, which state that quality cannot be tested into products; it should be built-in or should be by design. Within these QbD guidelines, the FDA has devised its PAT initiative. PAT is an industry-wide effort to understand and optimize every step of the pharmaceutical manufacturing process, by developing monitoring capabilities that enable timely control. The ultimate aim is to enhance production efficiency, quality, and safety by: reducing production cycle times; minimizing batch rejection; enabling real-time release; increasing the use of automation; and facilitating continuous processing. QbD and PAT both set out with the aim to improve quality and reduce production costs.
Current Methods of Fermentation Monitoring
It is necessary to determine the physiological condition of the cells, within a fermentation reaction, in order to fully monitor its status. One commonly used technique to control the production process is following the O2 consumption and the levels of CO2 emission measured by gas analysis in the head space of the fermenter. The ratio of these two is the Respiratory Quotient (RQ), which is a well-defined parameter for monitoring biological activity as it is dependent on the nutrient source that is being metabolized. However, it is an indirect measurement and also requires the use of mass spectrometry to provide suitable accuracy. Therefore, in order to provide a more robust measure of the progress of a fermentation reaction, the industry requires a real-time solution that directly analyzes the fermentation media, is easy to use, relatively inexpensive, and robust enough to withstand continuous exposure to the media.
NIR Monitoring
Near-infrared (NIR) absorption measurement is one of several spectroscopic techniques, which can be provided via a fiber optic inserted as a probe into the fermentation vessel. With the probe immersed in the fluid the spectrometer can gather in situ data about the progress of the reaction. Even though NIR provides a lot of chemical information, the current designs create a relatively expensive technique, and the development of the process model is highly complex due to the overlap in the absorption bands of the numerous analytes. Once this model has been generated, live data needs to be collected and analyzed by making comparisons with it. Full interpretation of the NIR data can be complex and, combined with its relative expense, may exclude it from the remit of many bioprocessing facilities.
SSRI Monitoring

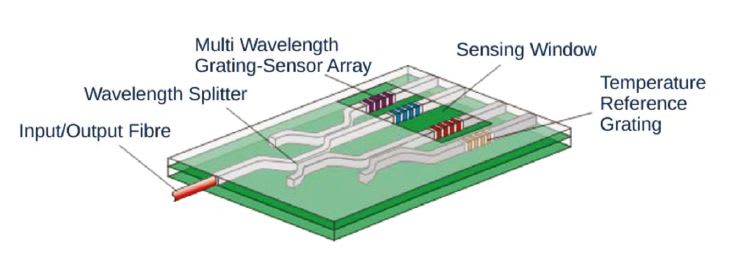
The solid-state sensor is designed on the concept of integrated optical circuits. Broadband light from the SMU is coupled into an optical fiber, which is connected to the microchip sensor. Light is reflected back from the sensor to the SMU and converted into a measurement- specific response. Within the microchip, the light is routed and channeled into integrated Bragg gratings, which only reflect specific wavelengths (Fig. 2). The specific wavelength of the silicon Bragg grating is very sensitive to changes of the refractive index (RI) on top of the chip. The sensor detects fermentation-specific parameters as well as changes of the temperature through changes of the RI of the liquid that is in contact with the sensing window of the microchip (Fig. 5).

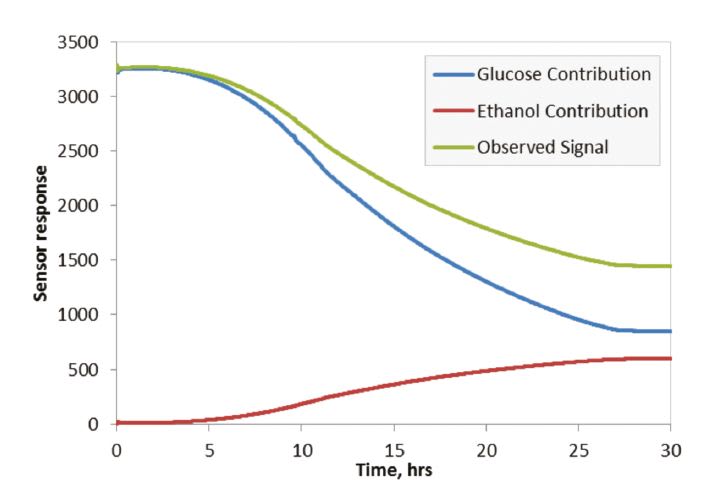
Figure 4 shows the corresponding concentrations derived from the measured response data. For comparison, glucose concentration measured by a conventional offline method outside the fermentation vessel is also plotted in this graph and shows correlation of real-time and offline data.
The simplicity of the system, with its easy-to-understand read-outs, means the data can be acted upon immediately without further analysis from a highly qualified scientist and the attendant time delay. The optical microchips are inexpensive to produce and as such can be disposable, making them increasingly relevant as moves are made toward single-use bioreactors (SUBs). The small form factor and separation of sensor for the analysis engine means that they can be used in lab-based development reactor vessels as well as full production bioreactors. Using the same sensing technology allows for correlation of data between systems during process transfer and scale-up. The new system is currently in trials and being integrated by process developers, and equipment and product manufacturers.
Conclusion Real-time online monitoring of key parameters of biopharmaceutical fermentation processes offers greater process control — leading to important cost savings and increased efficiencies. Achieving this requires the introduction of additional process analytical tools such as the SSRI technology, which is easy to integrate, economic to produce, and straightforward to use. The ability to monitor glucose levels in real time is a significant step forward in biopharmaceutical process control.
This article was written by Sam Watts, B.Eng, Ph.D., Co-founder and Business Development Officer for Stratophase Ltd., Hampshire, United Kingdom. For more information, e-mail



