It has been demonstrated that after a stroke-like lesion in the cerebral cortex of non-human primates, the remaining intact tissue undergoes extensive neuro-physiological and neuroanatomical remodeling. The ability of cortical areas remote from the infarct to form new cortico-cortical connections over long distances between the frontal and parietal lobes has been demonstrated. It is likely that these novel connections play a role in functional recovery after cortical injury.
This work combines neurobiological tools with implantable device technology to develop a novel microsystem to guide post-injury axonal sprouting in order to reshape cortical connections, optimize connectivity patterns, and improve long-term functional recovery after a traumatic brain injury (TBI). Advancements in designing neuroprosthetic devices to temporally couple the activity of remote cortical locations that are not normally coactivated or interconnected were used, taking advantage of the injured brain’s ability to produce growth-promoting substances that encourage anatomical rewiring. Such temporal coupling will encourage growing axons to migrate toward and terminate in the coupled region.
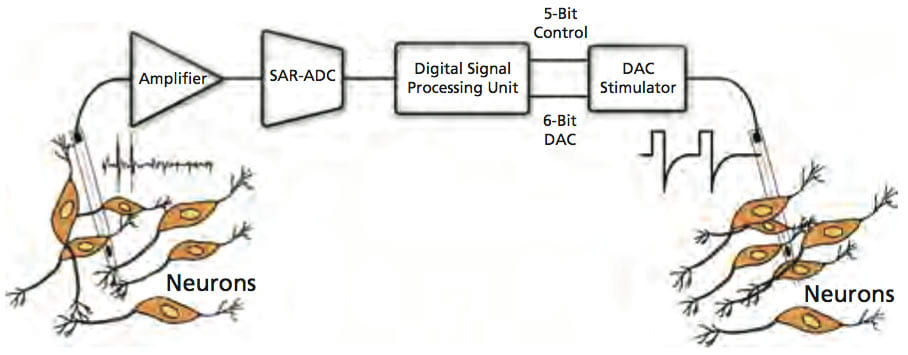
A fully integrated neural recording front-end comprising a low-noise, two-stage amplification circuitry and a 10-bit successive approximation register (SAR)-based analog-to-digital converter (ADC) was fabricated using the TSMC 0.35 μm 2P/4M n-well CMOS process. The ac-coupled amplification circuitry provided a maximum mid-band ac gain of 52 dB and featured a measured input-referred voltage noise of 3.5 Vrms from 0.1 Hz to 12.8 kHz, while dissipating 78 μW from 2 V. The SAR ADC featured an effective number of bits (ENOB) of 9.4 for maximum sampling frequency of 45 kSa/s, while dissipating only 16 μW.
Monolithic circuitry was designed using standard digital cells to identify the presence of large action potentials in the recorded digitized data with a spike discrimination algorithm based on two programmable threshold levels and time-amplitude windows. Detected spikes were then used to trigger the back-end microstimulator with a programmable delay. A first-order digital high-pass filter was also designed to remove any dc/low-frequency signal components prior to spike discrimination. The measured power consumption was less than 1 μW with a 1.5-V supply and ADC sampling frequency of 35 kSa/s.
A high-output-impedance current microstimulator was fabricated using the TSMC 0.35 μm CMOS process. It delivered a maximum current of 94.5 μA to the target cortical tissue with current efficiency of 86% and voltage compliance of 4.7 V with a 5-V supply. The stimulus current could be programmed via a 6-bit digital-to-analog converter (DAC) with accuracy better than 0.47 LSB.
A cross-coupled, voltage-controlled oscillator (VCO) for digital frequency shift-keyed (FSK) transmission based on an earlier design was developed. The oscillator tank employed a surface-mount, high-Q, off-chip inductor for low-power operation at a frequency near 433 MHz. The tank varactors were implemented using PMOS capacitors with source/drain/bulk connected to each other. To have flexibility in selecting a suitable _F for the FSK transmitter, the varactors were divided into two sets of binary-weighted capacitors that could be externally controlled with three bits. Using this tuning scheme, _F could be varied in the range of 2-14 MHz in steps of 2 MHz.
Rapid progress is being made toward developing smart prosthetic platforms for altering plasticity in the injured brain, leading to future therapeutic interventions for TBI that are guided by the underlying mechanisms for long-range functional and structural plasticity in the cerebral cortex. This first-generation integrated device was tested successfully in an anesthetized rat model by recording neural spikes from the somatosensory cortex of the brain, and stimulating the primary motor cortex that resulted in clear wrist movements. Behavioral assessments of reaching, retrieval of small food items, and locomotion demonstrate that deficits persist during the five-week recovery period following injury. Work is still ongoing for system assembly and packaging in the form of a miniature implantable device to test the system on long-range intracortical connectivity formation post-TBI.
This work was done by Pedram Mohseni, Ph.D., of Case Western Reserve University for the Army Medical Research and Materiel Command. For more information, download the Technical Support Package (free white paper) at www.medicaldesignbriefs.com/briefs . ARL-0103
This Brief includes a Technical Support Package (TSP).

Implantable Microsystems for Anatomical Rewiring of Brain Circuitry
(reference ARL-0103) is currently available for download from the TSP library.
Don't have an account?
Overview
The document is an annual report detailing a research project titled "Implantable Microsystems for Anatomical Rewiring of Cortical Circuitry: A New Approach for Brain Repair," led by Dr. Pedram Mohseni at Case Western Reserve University. The report, dated March 2009, covers the project's progress from March 1, 2008, to February 28, 2009, under the sponsorship of the U.S. Army Medical Research and Materiel Command.
The primary objective of the research is to develop implantable microsystems that can facilitate anatomical rewiring of cortical circuitry, which is crucial for brain repair, particularly following traumatic brain injuries (TBI). The report highlights the successful testing of a first-generation electronic microsystem in an anesthetized rat model. This system was capable of recording neural spikes from the somatosensory cortex and stimulating the primary motor cortex, resulting in observable wrist movements.
A significant aspect of the research involved inducing TBI in the rat model using a controlled cortical-impact device. This method allowed the researchers to create a targeted injury in the caudal forelimb area while sparing the rostral forelimb area, which was designated for the implantation of the electronic microsystem. The study demonstrated that various impact parameters could be employed to meet the experimental requirements for post-TBI implantation.
Behavioral assessments conducted during the recovery period revealed persistent deficits in reaching, retrieval of small food items, and locomotion over five weeks following the injury. These findings indicate a critical time window for testing the effects of the implanted device on recovery and rehabilitation.
The report also includes a section on reportable outcomes, listing several manuscripts, abstracts, and presentations resulting from the research. Notable contributions include works presented at various conferences, such as the IEEE Biomed. Circuits and Systems Conference and the Society for Neuroscience Meeting.
Overall, the document outlines a promising approach to brain repair through the development of implantable microsystems, emphasizing the potential for enhancing neuroplasticity and rehabilitation following brain injuries. The research aims to contribute significantly to the field of neuroscience and improve therapeutic strategies for individuals affected by neurological damage.



