
Artificial intelligence (AI), augmented reality (AR), virtual reality (VR), remote treatments, smart wearables, robotic mobility, and automation. Next to the ongoing evolution of evermore precise images and real-time videos, as well as evermore mobile and compact machines, the recent communication megatrends shift medical technology into the next level of healthcare digitalization.
What needs to be considered when making the connecting infrastructure and cable management ready for next-level data flow with extended mobility? The growing dimensions of today’s connected environment repeatedly challenge the status quo of approved connectivity components. This article identifies the major impact on the network infrastructure components itself: the cabling.
Connectors establish the contact; cables make the miles and movement. Counting the volume, cable systems represent one of the biggest shares when it comes to the components inside of many diagnostic imaging devices.
Benefits and opportunities arising from the use of AI, AR, or VR in medical applications are many and varied. Despite regulations for secure use and other immaterial needs to be established, the request for data-intensive exchange brings up new challenges to the cables themselves. Not only more bandwidth is needed, but it often has to be in line with extended reliable mobility or more compact design of devices.
What is important, if medtech designers want to create an AI-ready medical network infrastructure referring to the internal wiring of a medical device or the connectivity and interoperability of a device in a sustainable network? It is and remains crucial that any connection must serve the application to the maximum level of safety and comfort.

This article presents a closer look at five key factors that influence cabling in the future-proof connectivity of medical technology. An exemplary case that describes the particular challenges is intended to further illustrate the insights gained. In this example of cabling a hypothetical omnidirectional mobile C-arm imaging device, high-resolution real-time videos were to be supported with AI-generated image control. The application best illustrates the findings.
Extended Bandwidth: Is Fiber an Option?
Increasing data rates quickly lead to the conclusion that bandwidth-heavy connections should be implemented or upgraded with fiber optics — simple and elegant. But is fiber the only option and should be preferred to copper? As always, the reasonable upgrade or replacement of copper with fiber optic connections depends on several factors. Many advantageous features of fiber connections are clear. They have smaller diameters, are lighter, and do not require electromagnetic interference (EMI) shielding. However, regarding mobility and adaptability to environmental conditions, most standard fiber optic patch cables require improvements.
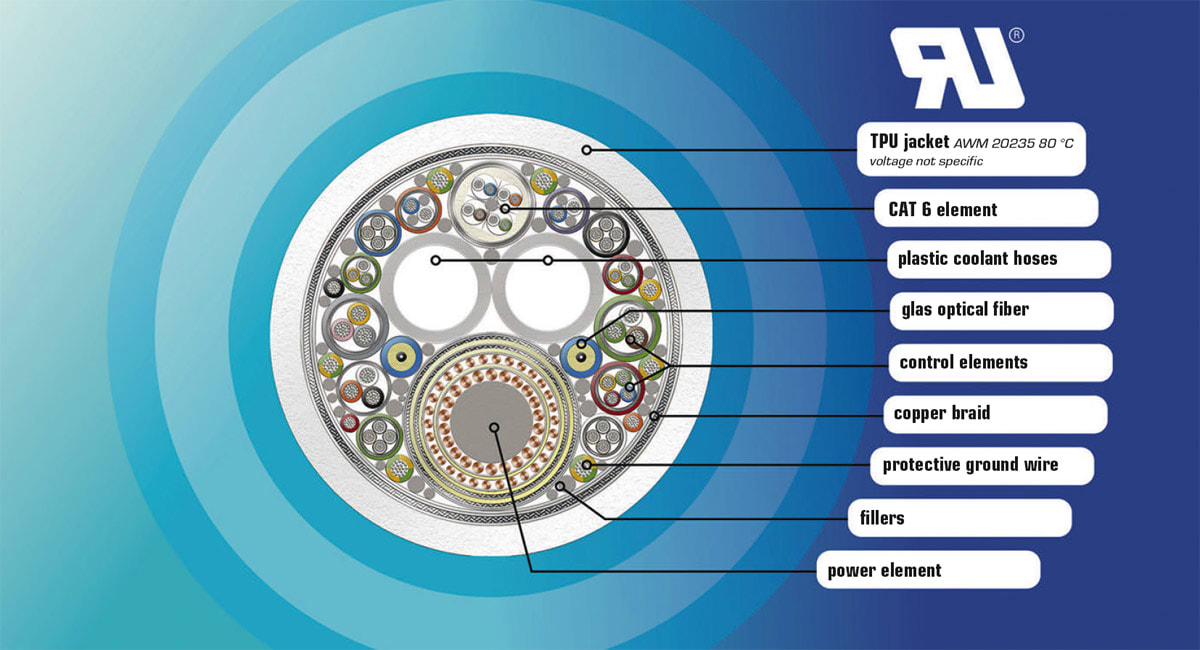
Reliable Cat, DisplayPort, HDMI, or radio-frequency (RF) copper cables need to be reinforced for frequent dynamic movements like bending, torsion, or both simultaneously as well. To make fiber optic and copper cables durable under frequent flexion or torsion, special insulation or jacket materials must be extruded alongside flexible fibers or stranded wires. Further strain relief elements need to be integrated into the bulk cable and the fiber optic assembly. This lastingly improves flexible cable routing and compliance with international standards. Application-optimized jacket materials equip any cable for better resilience against liquids like disinfectants or oils.
Hybrid cables — optionally incorporating fiber optics, twisted pair, coaxial, and power cables including media hoses — can provide the best balance between data rates and mobility. If designed and produced with sliding internal cable jackets and insulations, with the appropriate lay length of stranding of the many twisted wires, as well as fillers, separators, and other special construction elements, they can be the breakthrough for many compact and mobile construction designs. Last but not least: Fiber elements are additionally protected against mechanical and dynamical stress inside the tight hybrid cable bond.
Case Study: C-Arm Imaging Device
Optimal Cable Design. Now, what does all that mean when applied to the C-arm case? Achieving an optimal cable design for a compact mobile C-arm system integrating a flat detector with fiber optic transmission and copper-based power and signal lines presented several technical challenges. A key issue was a need to accommodate multiple cable types — fiber optics for increased bandwidth, twisted-pair and coaxial cables for high-speed signal integrity, and power lines — all within the constrained space of a jointed, moving system. Initial solutions using separate reinforced cables led to excessive stiffness and space constraints, particularly in tight bending radii and feedthrough points.
The accumulation of multiple protective layers, shielding meshes, and additional mechanical reinforcements created bottlenecks in critical articulation points, increasing stress on motors and limiting movement precision. By shifting to a hybrid cable design, the integration of fiber optics, signal, and power conductors into a single, EMI-protected sheath significantly reduced the total diameter, eliminating unnecessary cable bulk and slack while maintaining durability and flexibility.

Advanced materials and structured cable layering ensured reliable performance under continuous bending and torsion cycles. Furthermore, breakout sections allowed selective routing of specific elements to different extensions of the device, optimizing cable management without compromising system efficiency.
Sustainability. Besides application-optimized hybrid cables, as well as mechanically strengthened fiber optic and copper cables, planning and analysis of the most effective cable routing layout is the best tool to translate design targets to all cable components. It incorporates electrical, mechanical and dynamical stress analysis, early identification of critical points for the bulk cables, and opens up possibilities to make all connections more efficient, reliable, and easier to maintain over the system’s life cycle. Because hybrid cables have smaller diameters compared to cable bundles, they facilitate internal airflows and minimize ventilation efforts and energy consumption.
Consequently, special cables are strengthened as resilient components to survive stress analysis in customized movement tests and approve stability. Next to strain relief and cable management apparatuses, individual moldings offer a comparably easy way to protect and strengthen system compounds to harmonize mechanical loads. Additionally, the integration of molded connections can favor modularization for easy and sustainable maintenance. So, sustainable design implies the integration of lasting components, where they are needed. If still the likelihood of rapid wear and tear is identified for a certain section, or massive hygienic demands require disposable solutions, cable system modularization is a sustainable option.
Looking at the C-arm use case, what was done in addition to resilient cables to meet targets for greater sustainability? In addition to compliance with material selection, environmental policy regulations, and source control management (SCM) life cycle, lasting resilient components and power-efficient ventilation were already aligned with the customer’s sustainability goals for the new medical device series. The use of modular system architecture, RoHS-compliant materials, and power-efficient designs further supported these objectives. By reducing cable resistance and optimizing power distribution, energy loss and heat generation were minimized, contributing to the overall efficiency and sustainability of the system.
Another critical design aspect emerged at the detector end of the system. The identified challenge involved a 90° cable bend leading into the flat detector, which rotated during operation. Due to the high mechanical stress at this junction, an extra-molded cable fixture was introduced to secure the bend while maintaining slide ability and preventing abrasion against surrounding components. A modular approach was implemented by incorporating contact points before and after this section, along with a dedicated maintenance slot in the housing added by the customer.
This design allowed for localizing cable replacements without dismantling the entire system, significantly reducing electronic waste and minimizing service downtime. Since this section remained prone to wear, the cable system itself was also modularized — interchangeable segments with integrated interconnects enabled fast, precise replacements. Color coding and clear markings ensured easy reassembly. Next to the easy-to-exchange cable fixture module, a long-term tested cable storage unit facilitated smooth, uninterrupted detector rotation in the next section. By prioritizing durability and repairability, the solution not only improved operational efficiency but also contributed to a more sustainable product life cycle.
Safety: Electrical, Mechanical, Hygienic, Nontoxic
Medical device cables must comply with a range of international standards to ensure safety and performance. While UL, VDE, IEC, ISO, CE, USP, CSA, AWM, NEC, and FDA regulations all play a role, their relevance varies by application. UL standard 758 for Appliance Wiring Material (AWM) applies to cables inside a device, whereas cables installed within a building’s structure must be listed according to different UL safety standards and comply with relevant articles of the National Electrical Code (NEC). For patient-contact cables, biocompatibility standards like ISO 10993 and FDA guide-lines take priority. While VDE and IEC define the minimum requirements for medical devices in Germany and Europe, UL and CSA standards are stricter for the North American market and have become a benchmark for product safety in many other countries.
Manufacturers must ensure that cable designs align with their designated UL classifications, covering voltage, temperature limits, and material properties. In specialized environments like hybrid operating rooms, cables must also be sterilizable and biocompatible, minimizing risks associated with material composition. 20 different risk assessments of DIN ISO 10993 and six classes of the USP (United States Pharmacopeia Convention) are distinguished. The FDA also assesses the compatibility of a sheath material.
The specific construction of a cable system within medical devices must conform to existing standards. UL regulations provide general guidelines, while AWM Styles offer detailed specifications for certain applications. A UL-recognized cable manufacturer can qualify for different Style numbers and mark compliant cables with the “UL recognition mark.” Cables belonging to an AWM Style must be used in applications according to the Style description, which includes voltage rating, maximum operating temperature, conductor size (AWG), flammability class, and insulation and jacket materials with permissible wall thicknesses. It is crucial during development to ensure that the parameters of the applicable UL Styles align with the actual operating conditions.
In addition to meeting these existing standards, the increasing power and data flow requirements of AI technologies in medical devices may necessitate the development of new UL Styles specifically for device cabling. For example, new styles may be required to address the increased heat generated by high-speed data transmission or to ensure the reliability of cables that are constantly flexed or twisted by robotic equipment. By working with a UL-recognized cable manufacturer and staying informed about emerging UL standards, medical device manufacturers can ensure that their products meet the highest safety and performance standards while keeping pace with technological advancements.
Medical Device Development. What about the C-arm medical device development use case? What regulations were applied? As the hybrid cable designed to connect the C-arm flat detector was guided externally through a housing feed-through inside the unit and then split into different elements, UL Style 20235 had to be applied for external use cables. The maximum temperature of this AWM Style is 80° C; voltage is not specified.
Given the C-arm’s external guidance and free-range movement, the hybrid cable also needed to meet biocompatibility standards, as the cable could potentially come into direct contact with patients and medical staff. To achieve this, a dual-jacketed design was implemented, where the inner layer provided mechanical reinforcement, flame resistance, EMI shielding, and torsion resistance, while the outer jacket ensured compliance with medical-grade safety regulations. The biocompatible outer layer was composed of skin-safe, sterilizable thermoplastic polyurethane (TPU) resistant to disinfectants, preventing contamination risks in clinical environments. This design not only increased the cable’s durability in dynamic use but also ensured adherence to strict medical safety standards, offering a reliable, future-proof solution for advanced mobile imaging technology.
Finally, a special convex-shaped grommet made of lastingly disinfectable TPU ensured a seamless and hygienic connection with the device housing at the feed-through point.
Conclusion
The lack of compatibility between high electrical and highly dynamic requirements in cable design is not solved but taken to a new level with medical megatrends such as AI, robotics, and miniaturization together with indispensable sustainability and safety demands.
To permanently implement the benefits of emerging AI, extended reality (XR), remote functions using high-speed and miniaturized connectivity solutions in highly mobile applications, it is more crucial than ever to not only design the harnesses tailored to specific machine architectures, but better the cables themselves.
Involving an expert in the early development stage opens up possibilities to further create the cabling according to what is needed. Thus, more bandwidth, more mobility, more compactness will lead to more specialized, optimized and tailored cable solutions to respect all the growing dimensions of medical technology evolution.
This article was written by Ronald Spranger, Director of Business Development, BizLink Technology, Inc., El Paso, TX. Contact Spranger at



