
The global medical device manufacturing industry is undergoing a rapid transformation driven by technological innovation, automation, and increasing demands for customized, high-quality care. For engineers at the heart of medtech manufacturing, understanding the latest technologies is crucial not only for maintaining competitiveness but also for ensuring regulatory compliance, improving time to market, and optimizing production workflows.
This article provides an overview of key technological advancements reshaping the medical device manufacturing landscape, including additive manufacturing, robotics, laser welding, and more.
Additive Manufacturing: Redefining Production Flexibility
Additive manufacturing (AM), commonly known as 3D printing, is rapidly evolving from a prototyping tool into a core production technology within medical device manufacturing. It enables manufacturers to build highly complex geometries that are often impossible or prohibitively expensive using traditional subtractive methods.
Engineers are increasingly turning to AM to produce patient-specific devices such as orthopedic implants, dental crowns, and surgical instruments. Materials like titanium alloys, cobalt-chrome, and bioresorbable polymers are widely used in AM to enhance device performance and biocompatibility.
According to recent projections, the global 3D printing market in healthcare is expected to exceed $6 billion by 2027, high-lighting the rapid expansion of its role in clinical and manufacturing environments. In 2024, FDA issued new guidelines supporting performance-based standards for 3D printed medical devices, streamlining validation processes and helping engineers reduce product development timelines. StartUs Insights lists additive manufacturing among the top 10 manufacturing innovations (see Figure 1).
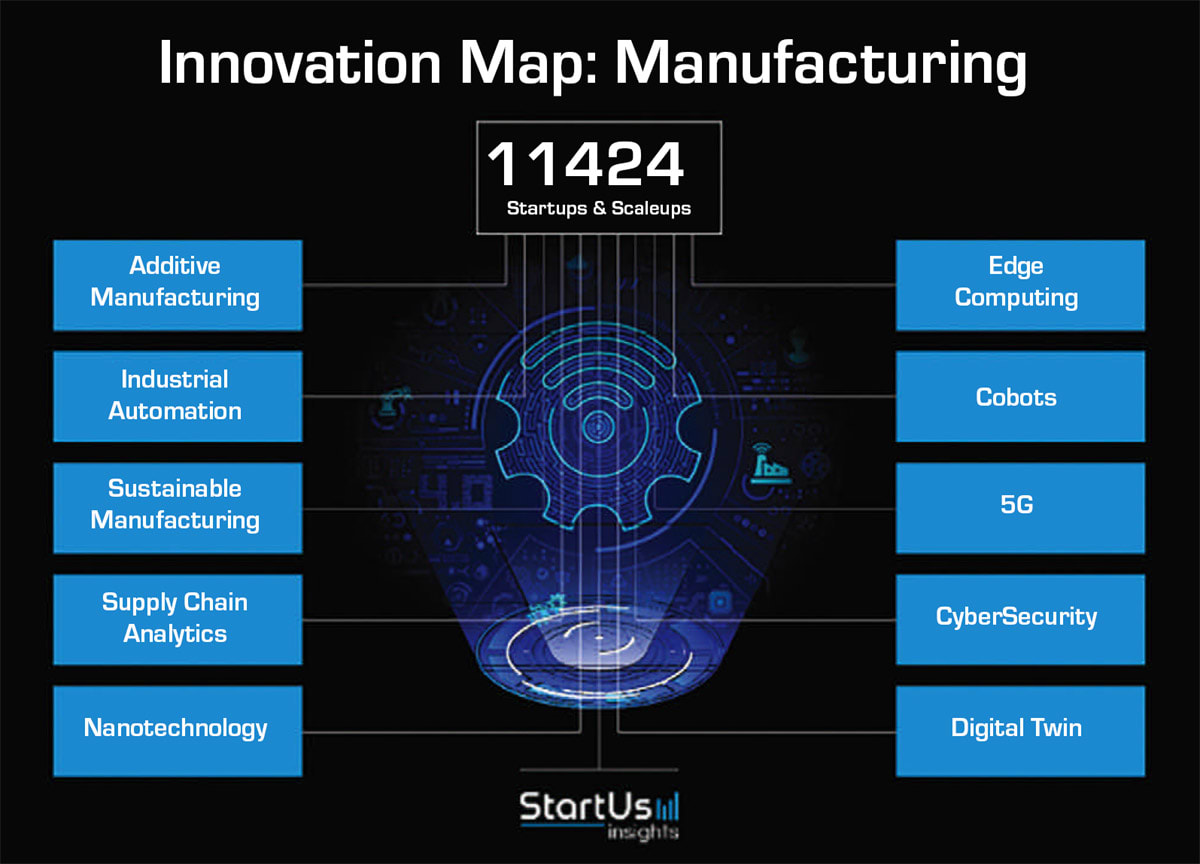
Dynamic Interface Printing: A Next-Gen Fabrication Frontier
Dynamic interface printing (DIP) is an emerging technology that leverages acoustic fields to manipulate materials at the air-liquid interface, enabling the printing of microscale, multimaterial, and biocompatible structures with remarkable precision.
In a recent article, researchers demonstrated DIP’s potential for fabricating flexible microelectrode arrays and implantable sensors. 1 The low thermal load and high-resolution output make DIP particularly attractive for applications requiring delicate features, such as drug-delivery nozzles or microfluidic channels in diagnostics.
Unlike conventional AM, DIP offers a hybrid approach that combines aspects of inkjet printing, stereolithography, and soft lithography, giving engineers a new level of control over spatial resolution and material heterogeneity. 1
Robotics and Vision Systems: Automating for Precision and Scale
Today’s medtech production lines rely heavily on robotic systems for both macro and micro scale tasks. These systems ensure consistent quality, improve yield, and reduce human error in repetitive or hazardous operations.
Collaborative robots (cobots) have become integral in automating tasks such as device assembly, welding, packaging, and labeling. These robots are designed to safely interact with human workers while maintaining high throughput rates. 2
At the same time, vision systems now utilize AI-driven algorithms to detect microscopic defects in real time. Engineers can train these systems using libraries of acceptable and unacceptable images, greatly enhancing quality control and documentation processes. Integration of vision systems with robotics also enables dynamic path correction, reducing downtime and scrap.
Engineers implementing robotic systems must address system validation, integration with MES/ERP platforms, and real-time data synchronization across production cells.
Laser Welding: Powering the Future of Medical Manufacturing
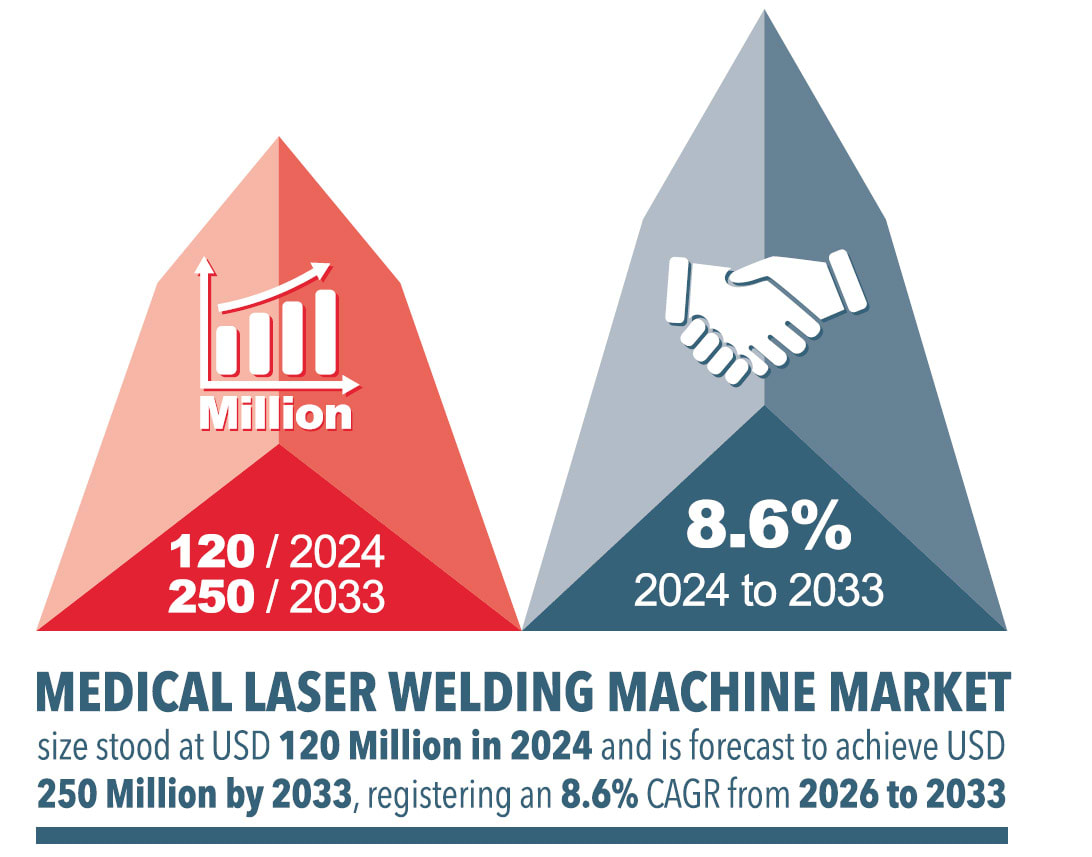
Laser welding is playing an increasingly important role in medical device manufacturing, offering a clean, precise method for creating strong and reliable joints. It is particularly effective in joining the intricate and/or miniaturized components found in devices such as intravenous lines, implants, and diagnostic tools — areas where traditional joining methods often fall short. Unlike adhesives or ultrasonic welding, laser welding produces hermetic seals without particulates or residues, helping manufacturers meet regulatory requirements while ensuring long-term reliability.
The technology is also compatible with a wide range of medical-grade materials, including a variety of polymers, glass, and metals, and it can join both similar and dissimilar materials. Its precision and repeatability also make it ideal for integration into automated production lines, enabling manufacturers to scale efficiently. Sales of laser welding systems continue to rise, as shown in Figure 2, underscoring the technology’s growing role in advancing quality and innovation in medical manufacturing.
Artificial Intelligence: From Design to Floor Optimization
Artificial intelligence (AI) and machine learning (ML) are transforming the medical device value chain. In manufacturing, AI tools are used to:
Predict equipment failures before they cause production downtime.
Monitor and enforce quality control via image analysis and anomaly detection.
Forecast supply chain disruptions and optimize raw material use.
Advanced ML models can process sensor data from IoT-enabled machinery to recommend adjustments in real time, improving yield and reducing waste. Additionally, AI-powered generative design tools allow engineers to input performance constraints and automatically generate compliant, optimized geometries.
This accelerates time-to-market and ensures alignment with evolving regulatory standards. As AI models become more sophisticated, their ability to simulate and validate device performance virtually will play a key role in reducing clinical trial and regulatory approval timelines.
Smart Manufacturing and IoT Integration
Smart manufacturing leverages IoT sensors, data analytics, and cloud computing to transform traditional factories into intelligent production environments (see Figure 2).
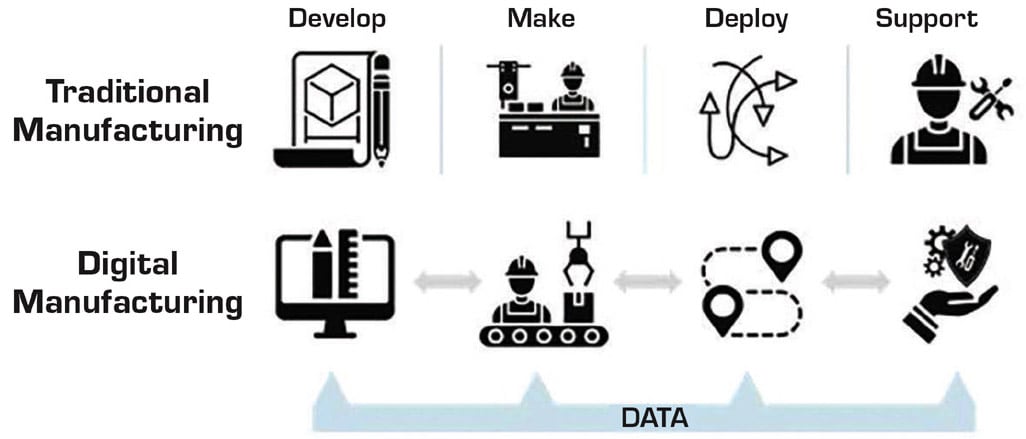
Sensors embedded in production equipment monitor parameters such as vibration, temperature, and torque. When this data is fed into edge or cloud-based platforms, it enables engineers to:
Perform predictive maintenance.
Trace device lineage for post-market surveillance.
Optimize batch sizes and reduce rework.
GlobalData forecasts that information and communication investment in medical manufacturing will climb to $31.7 billion by 2027, with significant portions allocated to IoT and real-time analytics. For engineers, the move toward smart factories also demands attention to cybersecurity protocols and interoperability standards. 3
Trends in Manufacturing Infrastructure
To meet growing global demand, medical device firms are expanding and upgrading their production facilities. These expansions often incorporate smart infrastructure, automation, and environmentally sustainable practices.
CMR Surgical’s 2024 opening of its 75,000-sq-ft UK production facility marks a pivotal shift. It houses production, quality, manufacturing engineering, supply, operations, and logistics. The site includes advanced robotics integration and quality assurance labs designed for high-throughput manufacturing of the Versius surgical platform. Similarly, Science Corp. recently invested in semiconductor-grade cleanroom facilities in North Carolina to support neural implant production.
New facilities are also deploying advanced equipment such as precision CNC machines, laser micromachining tools, and cleanroom automation systems. These tools enable engineers to meet tighter tolerances, scale production rapidly, and comply with international quality standards like ISO 13485 and FDA 21 CFR Part 820.
Challenges and Considerations
Despite the promise of advanced technologies, several barriers remain.
Regulatory complexity: Ensuring compliance with FDA, ISO, and MDR standards when integrating AI or AM requires cross-functional expertise and early regulatory engagement.
Cybersecurity risks: As manufacturing becomes more connected, ensuring end-to-end security is critical. The FDA and NIST both emphasize robust access control, endpoint protection, and intrusion detection. Listen to Medical Design Briefs podcast series “Addressing Cyber Threats and Safety in Medical Devices:
Best Practices for Securing Medical Devices
The FDA’s Role in Medical Device Cybersecurity: Regulations and Guidelines
The Growing Impact of Cybersecurity on Patient Safety in Medical Devices
AI and Machine Learning in Medical Device Cybersecurity
Skills gap: Engineers must be trained in new disciplines including data analytics, machine learning, and automation control.
Capital investment: Adopting new technologies often demands significant upfront investments in equipment, facilities, and systems integration.
Overcoming these challenges requires collaboration between engineering, IT, regulatory, and executive teams to ensure successful implementation and scaling.
Conclusion: Engineering the Future of Medtech
As the medical device manufacturing industry evolves, engineers are uniquely positioned to harness cutting-edge technologies that drive efficiency, quality, and innovation. From generative AI to dynamic interface printing, these tools provide a pathway toward faster development, safer devices, and more responsive manufacturing systems.
By investing in training, adopting smart infrastructure, and staying in line with regulatory trends, engineers can lead the transition into a new era of medical manufacturing that is more connected, intelligent, and patient-centric.
This article was compiled by Medical Design Briefs. The section and graphic on laser welding was provided courtesy of EWI. For more information, contact
References
- C. Vidler et al., “Dynamic Interface Printing,” arX-iv.
- B. Dillman, “Collaborative Robots Fill Automation Gaps For Medical Device Manufacturers,” Medical Design Briefs, October 2018.
- “Medical Devices Sector ICT Market Size and Forecast (by Country, IT Solution Area and Vertical) to 2028,” GlobalData, July 19, 2024.



