The promise of additive manufacturing (AM) in the medical device industry has always been clear, the ability to create intricate geometries, patient-specific implants, and previously impossible structures. The reality, however, is far less inspiring. Often, manufacturers believe they are designing for AM, but in truth, most have only scratched the surface of what is possible. They are working within the confines of traditional design principles and are often defaulting to software-driven solutions, believing these tools will carry them across the finish line.
But the brutal truth is this — if you are not truly designing for AM, you are not only missing opportunities, but you are also failing to achieve the full potential of the technology. Worse still, if you are relying on off-the-shelf AM design tools, you are operating within a false sense of security, thinking you have optimized a design when, in reality, you have only automated mediocrity.
What Is Design for Additive Manufacturing?
At its core, design for additive manufacturing (DfAM) is not just about ensuring a part can be 3D printed — it is about engineering the geometry, the structure, and the function of the part in a way that fully leverages the strengths of AM while avoiding its pitfalls. Some of the most basic ways to approach this include reducing the number of components in an assembly, optimizing standard lattice structures for strength and weight reduction, and orienting parts in a way that minimizes the need for support material. The advantages of such an approach are undeniable. It reduces material waste, improves performance, streamlines post-processing, and reaches entirely new levels of design freedom that conventional manufacturing cannot offer. For the medical sector, this means more lightweight yet durable implants, better-performing surgical tools, and the ability to customize devices to a degree that was once unthinkable.
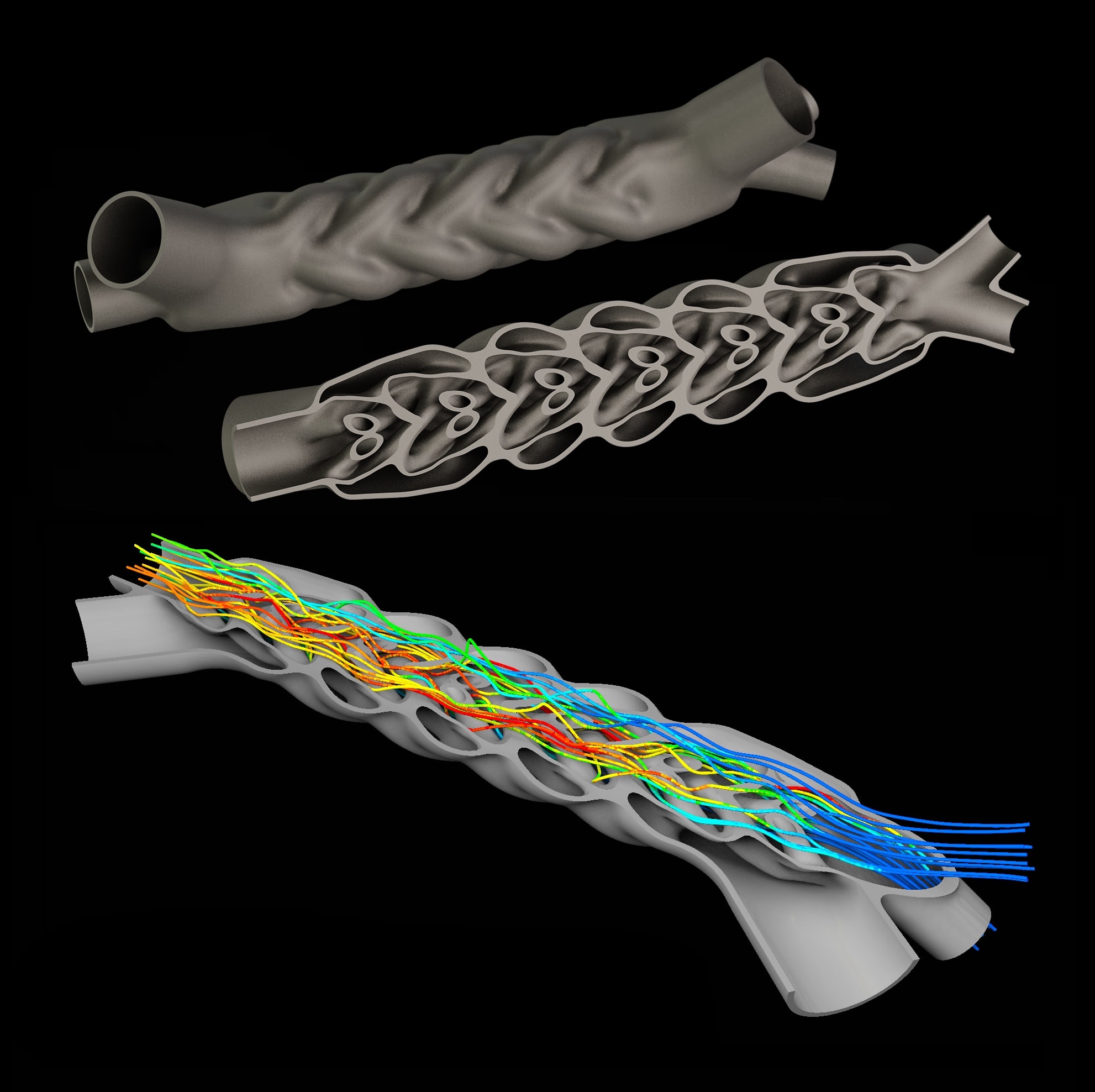
However, true DfAM does not stop at mere printability. The real power of AM lies in its ability to rethink how medical devices are structured, function, and interact with the human body. Consider, for example, the laborious design of implantable medical devices. A purpose-built computational design workflow could be used to facilitate the generation of form so that the implant blends with the surrounding tissue and matches the healthy side (see Figure 1).
By going a step further, the structure of such a device can be designed so that it actively encourages osseointegration through bio-inspired bespoke lattice designs that mimic natural bone. AM allows for controlled porosity, variable material densities, and intricate fluidic channels that traditional manufacturing simply cannot replicate. For drug-delivery applications, AM offers the ability to embed reservoirs and microfluidic pathways within implants, creating devices that not only provide structural support but also release medication at controlled rates over time. These are not theoretical possibilities. They are achievable outcomes, provided that the design philosophy is centered around the true potential of AM, rather than simply adapting traditional designs for a different manufacturing process.
Despite this, most medical device manufacturers approach AM with the same mindset as they do traditional manufacturing, expecting that software alone will guide them to the perfect solution. The rise of DfAM tools — such as Autodesk Netfabb, nTopology, Materialise Magics, and Siemens NX AM — has reinforced this notion. These programs are undeniably powerful. They can suggest complex organic geometries through topology optimization, automate lattice generation, and even analyze thermal distortions during printing.
But there’s a problem. These tools are only as good as the assumptions they are built on. Much like traditional CAD tools that replaced manual drafting, these software solutions simply swap conventional operations like extrusion and sweeping with generalized tools for latticing, ribbing, and topology optimization. They don’t generate novel geometry — they execute predefined geometric operations. They help navigate within known design spaces, but they will never push a designer into unexplored territory where true AM innovation lies.
Beyond Software-Led DfAM
And this is where the illusion of software-led DfAM begins to unravel. Software companies promote the idea that AM design can be solved with plug-and-play solutions, but the truth is that the medical industry needs bespoke solutions in response to nuanced clinical needs. Commercial software operates within predefined parameters, and true innovation in AM requires expert knowledge and the ability to break through these constraints.
This is precisely where Metamorphic’s approach to DfAM sets itself apart. Unlike traditional software-driven workflows, which rely on predefined design heuristics, Metamorphic treats each AM challenge as a completely new problem that requires a custom-built computational design and simulation strategy. The company does not simply use latticing tools to “fill in the blanks” — it develops bespoke algorithms tailored specifically to the application at hand. Every aspect of the geometry is engineered for purpose, rather than reverse-engineered to fit within a tool’s constraints.
For medical device manufacturers, this approach is transformational. It is not about tweaking an existing design for printability — it is about rethinking what a medical device can be when the constraints of traditional manufacturing no longer apply: A spinal
implant that functions as both structural support and active drug-delivery system. A surgical instrument that integrates biomimetic flexures for unparalleled dexterity. A prosthetic with adaptive mechanical properties, adjusting in real-time to patient movement.
These are not concepts that arise from a software-defined design space. These are outcomes that only emerge when true DfAM expertise, advanced computational modeling, and deep industry knowledge are applied in concert.
DfAM and the Future of Medical Design
A holistic approach to DfAM opens an entirely new world of possibilities for medical devices. Beyond merely optimizing support structures or reducing part count, it invites engineers to think in fundamentally different ways about how devices interact with patients. The flexibility of AM means multifunctional designs can be realized, where one device performs several roles instead of requiring multiple separate components.
One of the most powerful but underutilized aspects of AM in medical design is functional grading of materials — the ability to blend different material properties seamlessly within a single structure. This enables the creation, for example, of implants that combine rigidity and flexibility, allowing for better load distribution and reduced stress shielding, which often leads to implant failure in conventional designs. With traditional manufacturing, material transitions require bonding or assembly techniques that introduce weak points, but AM allows for a continuous gradient between materials, removing these limitations entirely.
Another major advantage of AM when properly leveraged is the integration of bio-inspired design principles. Nature has spent billions of years refining efficient, resilient, and adaptable structures, and AM finally allows engineers to incorporate these directly into medical device development. Trabecular structures in bones, vascular networks in organs, and micro-textured surfaces found in biological systems offer superior strength-to-weight ratios, optimized fluid dynamics, and enhanced biological integration. These concepts can be built directly into AM-designed medical devices, but only if the approach to design is not constrained by conventional thinking or limited to software-generated outputs.
Medical device manufacturers who lean heavily on off-the-shelf AM design tools are not just failing to optimize their products — they are actively limiting their competitive edge. The reality is that AM is no longer about whether it can be used in production, but how well it is used. And the companies that continue to rely on generic, software-driven approaches are only contributing to a cycle of mediocrity that holds the industry back.
Metamorphic challenges this stagnation by proving that truly optimized AM designs are only possible when creativity and expertise — not software — drives the process. The company does not just accept the outputs of generative design — it questions them and refines them. It does not settle for what can be printed — it engineers what should be printed, ensuring that every AM-produced medical device benefits from this unprecedented design freedom.
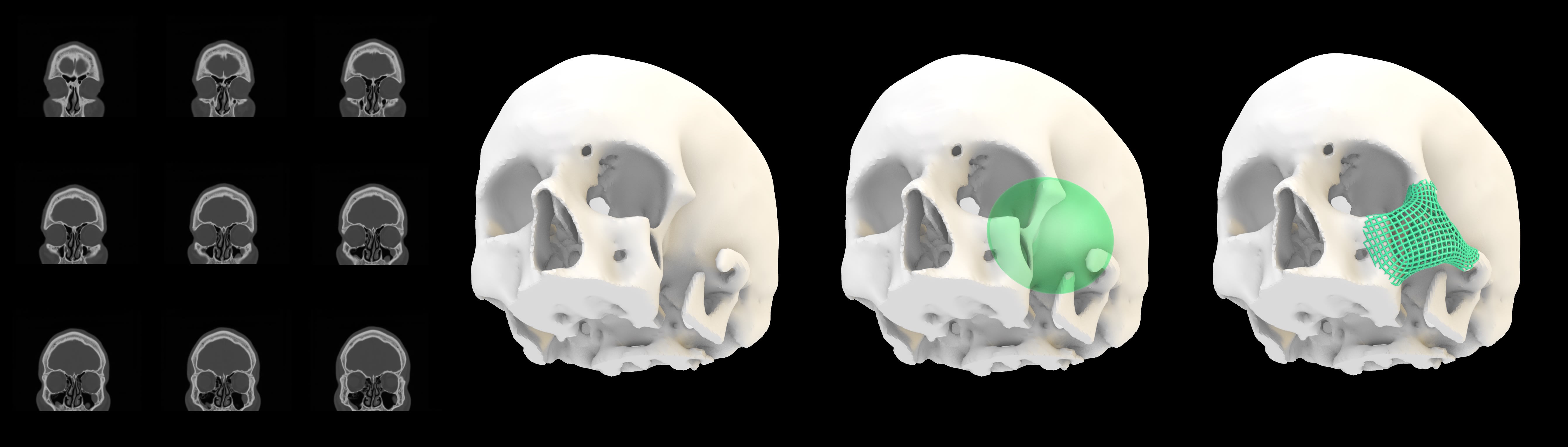
True optimization in medical device AM requires a combination of expertise, engineering intuition, and computational modeling that adapts to the unique challenges of each application (see Figure 2). The companies that succeed in fully harnessing AM will be those that go beyond the standard tools and design workflows, embracing a truly bespoke, first-principles approach to engineering every aspect of a device’s geometry, material composition, and functional performance.
For the medical sector, where precision, performance, and reliability are vital, this distinction is not just valuable — it is essential. The future of medical AM does not belong to those who simply “design for AM.” It belongs to those who design for AM the right way, with intent, with expertise, and with the courage to break free from the limitations of conventional thinking. This is the path that will define the next generation of medical device innovation.
Summary
For DfAM to move industry forward, the illusion that software-driven solutions alone can achieve true AM optimization should be dismantled, and instead a bespoke, first-principles
approach to engineering should be advocated. While many rely on automated workflows, true innovation in AM demands deep expertise, intent-driven design, and the ability to challenge conventional assumptions.
By focusing on custom computational strategies, functional material grading, bio-inspired geometries, and multifunctional integration, AM is pushed beyond simple manufacturability into entirely new realms of performance and functionality. This is not about making designs printable, it is about redefining what medical devices can be, ensuring that every aspect of an AM-produced component — from its mechanical behavior to its interaction with the human body — is engineered with precision, purpose, and optimization beyond the limits of traditional design constraints.
This article was written by Laurence Coles and Manolis Papastavrou, Founders, Metamorphic AM, Derbyshire, UK. For more information, visit here .



