Every year, 1.5 million Americans incur head injury; the wars in Iraq and Afghanistan have further increased this number. Head injury affects people of all ages and is the leading cause of death, long-term disability, and economic cost to society. While there are many aspects to head injury, the most urgent problem that must be detected and treated promptly within the first “Golden Hour” is brain bleeding, or an intracranial hematoma. Forty percent of head trauma victims on the battlefield develop brain bleedings.
Today, patients are sent to the closest hospital, which, in many cases, does not have neurosurgical service. In the event of a true hematoma, the patient must be transported to a trauma center that has neurosurgical coverage. This takes time, and time is the greatest factor in reducing the possibility of a neurological deficit or death.
CT scanning is the gold standard for identification and localization of traumatic intracranial hematomas. For those patients with neurological injuries, a CT scan, followed by surgery if necessary, is obtained immediately after the patient is hemodynamically stabilized. In rural areas of the U.S. or in underdeveloped areas of the world, timely identification of patients that require surgery can be difficult. Methods for identification of patients with hematomas in these settings are primarily the neurological exam and possibly the skull X-ray. However, both of the modalities are unreliable for this purpose. Due to high cost of CT, it is also not practical to scan a patient every couple of hours in an intensive care unit.
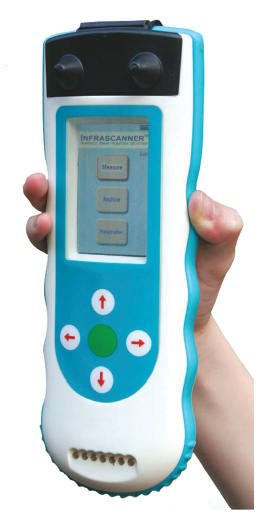
The Infrascanner was developed to address this need. This handheld non-invasive brain hematoma detector allows rapid screening as a CT scanner adjunct tool for monitoring patients within hospitals. It can significantly improve the way the health system identifies patients who need emergent neurosurgical intervention. The inventors of the Infrascanner technology are the father of using light in medicine, Dr. Britton Chance from the University of Pennsylvania, and a Neurotrauma guru from Baylor College of Medicine, Dr. Claudia Robertson. Together with an entrepreneurial team from Drexel University, they created InfraScan (Philadelphia, PA) in 2004.
How it Works

The basic method for hematoma detection is using Near Infrared (NIR) technology based on the differential light absorption of the left versus right brain. Under normal circumstances, the brain’s light absorption should be symmetrical. Where additional underlying extravascular blood is present, there is a greater local concentration of hemoglobin and consequently the absorbance of the light is greater while the reflected component is commensurately less. This differential is detectable via sources and detectors placed on symmetrical lobes of the skull.
The Infrascanner is a small, battery-operated, portable, and non-invasive handheld device that is intended as a screening device for “up-triaging” of patients with suspected hematomas for further CT scans and diagnostic workups. The system includes an 808-nm diode laser located 4 cm away from a silicon light detector, covered by a NIR optical filter. The laser delivers NIR light to the tissue under the sensor, via an optical fiber and the detector receives it via a separate optical fiber after it has interacted with the tissue. The 4-cm separation of light source and detector allows measurement of near-infrared light absorbance in a volume of tissue approximately 2 cm wide by 2 to 3 cm deep. The detector signal is then digitized and analyzed. A full head scan takes less than two minutes. The results would be expected to be a more accurate triage of patients that have intracranial hematomas, earlier surgery and as a result, a better outcome.
Where it Stands
The Infrascanner completed multicenter clinical trials in the U.S. and abroad. In 2008, InfraScan received ISO 13485 and the CE Mark, which is a license to sell in Europe and many other countries. In December of 2011, the FDA approved the Infrascanner Model 1000, the first handheld device intended to aid in the detection of life-threatening bleeding in the skull using near-infrared spectroscopy.
The FDA is specifying special controls in an accompanying regulation classifying the Infrascanner Model 1000 as a Class II device with special controls. The special controls provide information about specific risks that must be addressed by other manufacturers who may wish to market a similar device. Like many medical device companies, InfraScan launched international sales first, with a U.S. launch planned for 2012. The Infrascanner also conducted field evaluation with the U.S. Navy and Marines in Iraq and Afghanistan.
Near Infrared diffuse spectroscopy is an active research field. The Infrascanner is one of the first “low-hanging fruits” of this technology that completed the full path from bench to bedside. In addition to the application for detecting brain hematomas, future applications for the product include the monitoring of stroke victims.
More Information
For more information about the Infrascanner, visit http://info.hotims.com/40430-161 .



