Cervical Stabilization Device (CSD)
Benjamin Booher ViaTechMD,
Scottsdale, AZ
ViaTechMD has invented and developed the first and only device-based treatment for various conditions known to contribute to preterm birth (PTB). PTB is a growing global healthcare crisis and is the leading cause of infant death and morbidity within the first months of life. Nearly 3,000 babies die each day as a consequence of PTB, which is also recognized as one of the most significant contributing factors in several lifelong crippling diseases including autism, cerebral palsy, blindness, deafness, various respiratory ailments, and other cognitive and learning disabilities.
The latest publication from WHO (2018) reports that in 2014, approximately 10.6 percent of all live births globally were preterm. Every year, an estimated 15 million babies are born preterm (before 37 completed weeks of gestation), and that number is rising. Socioeconomic consequences of PTB are estimated to exceed $52 billion each year.
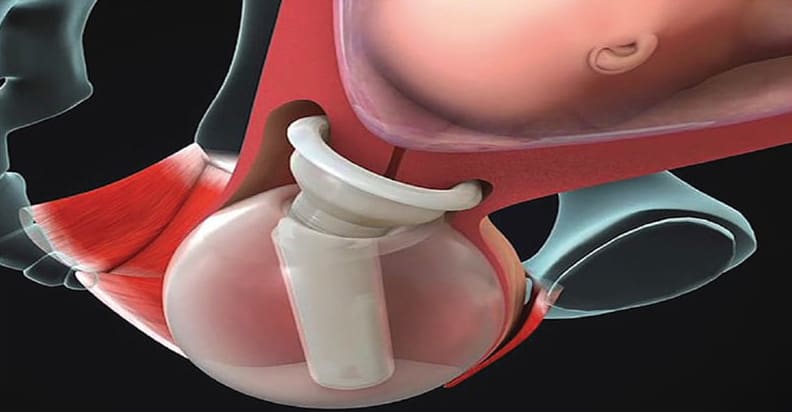
ViaTechMD’s globally patented device, the Cervical Stabilization Device (CSD), offers a cost-effective, noninvasive, medication-free, preventative treatment designed to support a successful and natural birth by addressing a wide range of conditions known to contribute to PTB (such as cervical incompetence, known cases of organ and tissue compromise, multiple fetus pregnancies, late-in-life pregnancies, and others).
Successful CSD human fitment studies took place in December 2016. The generation 6 CSD, in its near-commercial form, were slated to undergo a second round of human fitment studies in July 2019, ahead of pivotal FDA clinical trials that are expected to commence in the fourth quarter of 2019.
Current treatments in prevention of PTB include cervical cerclage (a costly, high-risk, invasive surgical procedure) and use of progestins (natural or synthetic female hormones that present a number of serious risks particularly to male fetuses). Neither treatment is effective in reducing bearing forces acting upon the uterine and cervical tissues that are known to accelerate the birthing, effacement and dilation processes. And, after decades of practice, neither treatment has demonstrated proof of efficacy.
To date, ViaTechMD has been operated as a virtual company and has therefore been very capital efficient. The company has raised approximately $3.5 million in “friends and family” equity capital and is seeking additional equity capital to fund a full-time management team, finalize product development, establish manufacturing operations in partnership with Freudenberg Medical, and to conduct a pivotal FDA clinical trial.
Videos of the technology are available here .
For more information, visit here .
HONORABLE MENTIONS
Monitoring of the Central Blood Pressure Waveform Via a Conformal Ultrasonic Device
Chonghe Wang, University of California San Diego,
La Jolla, CA USA
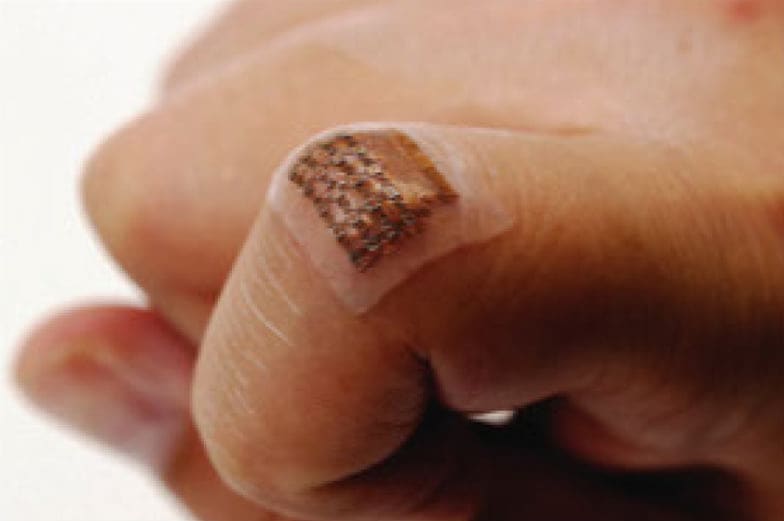
Continuous monitoring of the central blood pressure waveform from deeply embedded vessels such as the carotid artery and jugular vein has clinical value for predicting cardiovascular mortality. This ultrasonic device is conformal to the skin and capable of capturing blood pressure waveforms at deeply embedded arterial and venous sites. The wearable device is ultrathin and stretchable and enables the non-invasive, continuous, and accurate monitoring of cardiovascular events from multiple body locations, which should facilitate its use in a variety of clinical environments.
For more information, visit here .

Apparatus and Method for Donning Hygienic Gloves
Martin Harrison and Steve B. Kalish, HYGIENEXT, Chicago, IL USA
Healthcare-associated infections (HAI) occur in 15% of hospitalized patients in the US and result in approximately 90,000 deaths. Glove use is an attractive strategy because it is a highly effective measure for preventing HAIs. A method was developed that rapidly, easily, aseptically, and inexpensively dons hygienic, FDA-approved gloves with a single hand. Widespread use of this product could reduce HAIs, improve the quality of healthcare, and decrease morbidity and mortality.
For more information, visit here .
Biomimetic 3D-Printed Scaffolds for Spinal Cord Injury Repair
Jacob Koffler, Wei Zhu, Shaochen Chen, and Mark Tuszynski,
University of California San Diego,
La Jolla, CA USA
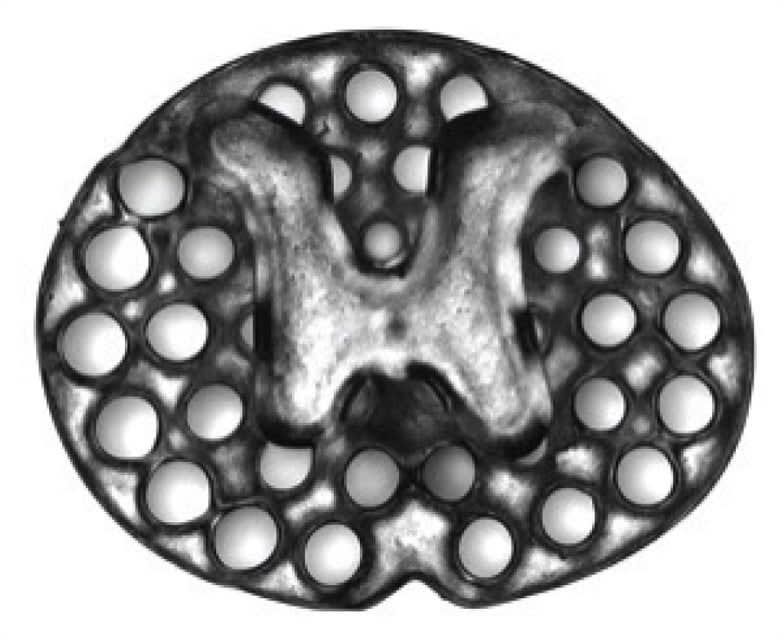
Natural spinal cord architecture was used as a template to design a scaffold that mimics the intact spinal cord. 3D printing technology can fabricate the implant in one continuous print, rather than layers, as with common inkjet 3D printers. The implants are loaded with neural stem cells that differentiate and create new neurons that can connect to the intact nerves in the spinal cord. Host nerves penetrate the implant and connect to the neural stem cells, transferring incoming commands (from the brain) to the target muscles.
For more information, visit here .
Body-on-a-Chip
Anthony Atala, Colin Bishop, Adam Hall, John Jackson, Sang Jin Lee, Sean Murphy, Tom Shupe, Aleks Skardal, Shay Soker, and James Yoo,
Wake Forest Institute for Regenerative Medicine,
Winston-Salem, NC USA
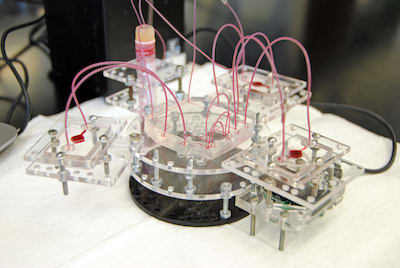
The Body-on-a-Chip platform is an accurate model of the structure and function of human organs such as the lungs, liver, heart, brain, etc. These micro-organs (organoids) are housed separately in small micro-reactors and connected by a microtube network that can feed them. It enables researchers to circulate candidate drugs, vaccines, or biologic agents or therapies between the different organs, as it would do in humans, to predict whether it is safe, effective, or toxic.
For more information, visit here .
See the rest of this year's winners:
- GRAND PRIZE WINNER
- ELECTRONICS/SENSORS/IOT CATEGORY WINNER
- MANUFACTURING/ROBOTICS/AUTOMATION CATEGORY WINNER
- AEROSPACE & DEFENSE CATEGORY WINNER
- AUTOMOTIVE/TRANSPORTATION CATEGORY WINNER
- CONSUMER PRODUCTS CATEGORY WINNER
- SUSTAINABLE TECHNOLOGIES CATEGORY WINNER



