News: Medical
A new report forecasting the growth of the Disposable Medical Devices Sensors Market to 2018 has been issued that says that the global disposable devices sensors market is technology driven and marked by the "threat of obsolescence," wherein technologies and their adoption change very rapidly. The...
R&D: Materials
A team of researchers from the University of California, Riverside, College of Engineering and School of Medicine have developed a novel transparent skull implant that could eventually lead...
Applications: Medical
Diabetes is a widespread metabolic disorder, and having it puts people at increased risk for heart disease and stroke. There are two types of diabetes patients: type 1...
Products: Materials
Parker Hannifin Corporation, Parflex Division, Ravenna, OH, offers TexLoc PTFE 4:1 Heat Shrink Tubing with the largest expansion ratio available in a PTFE tube. Operating in high temperatures up to 500°F, it...
Briefs: Test & Measurement
The microbead packing is the critical element required in the success of onchip microfabrication of critical microfluidic components for in-situ analysis and detection of chiral amino acids. In order for microliquid chromatography to occur, there must be a stationary phase...
News: Medical
All interested parties are welcome to join in the development of a proposed new ASTM International standard that will provide guidance on how to conduct axial, bending, and torsional fatigue testing of stents. The proposed new standard, ASTM WK23330, Guide for in vitro Axial, Bending and...
News: Regulations/Standards
Today, the FDA issued its final rule on the unique device identification (UDI) system that, once implemented, will provide a consistent way to identify medical devices. The UDI system should improve the quality of information in medical device adverse events reports, which will help the FDA identify product problems more...
Briefs: Materials
Bioresorbable polymers for medical devices encompass a broad class of materials with two of the more common materials being poly(L-lactic acid) and poly(lactic-co-glycolic acid). Some terminal...
News: Communications
The FDA Center for Devices and Radiological Health, Office of Science and Engineering Laboratories, Center for Biologics Evaluation and Research issued a Guidance document on “Radio Frequency Wireless Technology in Medical Devices” containing recommendations to assist industry and...
Mission Accomplished: Medical
Currently, in the United States alone, there are more than 10 million people whose movement is profoundly limited by diseases of and injuries to the brain and spinal cord. About half of these people...
Global Innovations: Medical
The Hong Kong Polytechnic University, Hong Kong, Chinahttp://www.polyu.edu.hk/cpa/polyu/index.php
Ateam of researchers in the Interdisciplinary Division of Biomedical Engineering (BME) at The...
Features: Regulations/Standards
Insulating and jacketing material options for wire and cable are innumerable, even if the field is narrowed to those with some qualification for use in medical electronics....
News: Medical
On July 9, the FDA issued a draft guidance on manufacturers’ responsibility to report adverse effects from their products: “Medical Device Reporting for Manufacturers,” for the purpose of seeking comments. Comments and suggestions should be submitted regarding this draft...
Products: Electronics & Computers
Mahr Federal, Inc., Providence, RI, announces the new Micro- Dimensionair® II, the next generation of its line of portable air gages, which incorporates an enhanced digital indicator and an...
INSIDER: Medical
Scientists at the Computer Science and Artificial Intelligence Laboratory at the Massachusetts Institute of Technology, Cambridge, have developed a new algorithm that, they say, can accurately measure the heart rates of people shown in ordinary digital video by analyzing the tiny head movements that go along...
News: Research Lab
Indiana Governor Mike Pence joined state-based global life sciences and research university executives to unveil the Indiana Biosciences Research Institute, the first industry-led collaborative life sciences research institute in the country. The Indiana Biosciences Research Institute is...
Products: Medical
Rinco Ultrasonics, Danbury, CT, a leading manufacturer of ultrasonic welding equipment, was awarded a US patent for its PPS0145 film sealing technology for ultrasonic film sealing of flexible packaging. This...
Products: Photonics/Optics
Edmund Optics, Barrington, NJ, introduces new TECHSPEC® Pre-Mounted Fluorescence Filter Cubes. These fluorescence filter cubes easily integrate into Olympus and Nikon microscopes for use in a range of life...
Products: Medical
Fluke Biomedical, Everett, WA, announced today the debut of its new VT305 Gas Flow Analyzer. The VT305 Gas Flow Analyzer features internal sensors, a four-button control panel, auto-orienting color LCD screen, and single...
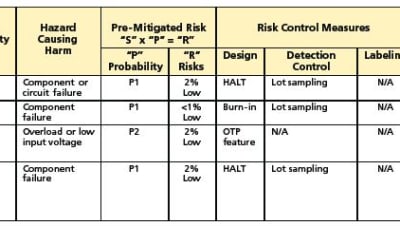
Features: Regulations/Standards
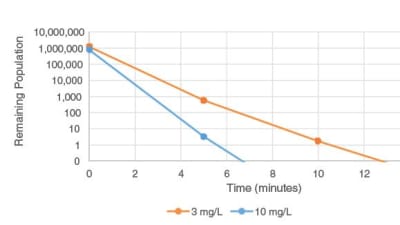
The recent publication by the U.S. Food and Drug Administration (FDA) of Guidance for Industry Pyrogen and Endotoxins Testing: Questions and Answers, dated June of 2012, the Department of...
Features: Medical
Now that the Centers for Medicare and Medicaid Services (CMS) has published its long-awaited final rule implementing the Physician Payments Sunshine Act (Federal Register, February 8, 2013), medical device manufacturers can move forward to carry out a familiar task: complying with regulations.
Products: Medical
Nameplates for Industry, Inc., New Bedford, MA, introduces custom domed labels that provide a durable and colorful alternative to injection molded or stamped 3D name plates. Featuring a high-gloss, non-yellowing,...
Products: Manufacturing & Prototyping
The Caplugs, Buffalo, NY, Class 8 clean room is ISO 9001 certified and recently received ISO 13485 recommendation. Caplugs caters to medical customers by providing traceability, record retention, and its strict...
Products: Packaging & Sterilization
Digicom Electronics, Inc., Oakland, CA, a technology and quality- driven electronics manufacturing services company, introduces its new Diamond Track Cleaning Process. This process uses a proprietary combination of...
Global Innovations: Robotics, Automation & Control
Chalmers University of Technology, Gothenburg, Sweden www.chalmers.se/en/pages/default.aspx
A team of researchers at Chalmers University of Technology in Gothenburg, Sweden, say that they have...
Applications: Electronics & Computers
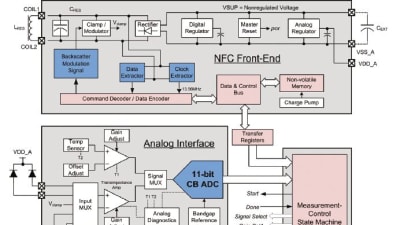
For medical device OEMs seeking compliance to the 3rd Edition of IEC 60601-1 for their power supplies, what is quite clear by now are the regional dates for enforcement,...
Features: Electronics & Computers
Electrical equipment used in medical technology must not place patients or medical staff in danger. This, in turn, requires that designing safe equipment starts at the...
Features: Manufacturing & Prototyping
No other industry in the US is under more pressure than medical electronics, and those pressures continue mounting each year for greater innovation and products that are...
INSIDER: Medical
Scientists at the Georgia Institute of Technology, Atlanta; the University of Alabama, Tuscaloosa; and Vanderbilt University, Nashville, TN, announced a four-year joint project to develop a below-knee prosthesis capable of actively powering the ankle joint powered by a gas- or liquid-based...

 Podcasts: Medical
Podcasts: Medical Podcasts: Design
Podcasts: Design Podcasts: Design
Podcasts: Design On-Demand Webinars: Medical
On-Demand Webinars: Medical Podcasts: AR/AI
Podcasts: AR/AI Podcasts: Manufacturing & Prototyping
Podcasts: Manufacturing & Prototyping