A lack of a specialized label is all it takes to halt the production of a medical device. While those of us in medical device manufacturing were aware of this fact, we became acutely experienced with scarcity and supply chain disruptions as a result of the global pandemic, including shipments of lifesaving medical devices delayed because of a quality issue and upstream material shortages. Now into the third year of the effects of the pandemic, the supply chain issues remain and may grow worse again as new disruptions present themselves.
In the past few years, as the vice president of business operations for a large medical device contract manufacturing company, I’ve spent countless hours in unplanned meetings chasing down raw materials, while collaborating across stakeholder functions to navigate missing resources and moving-target timelines. More recently, I’ve been working closely with materials suppliers and our customers’ procurement and engineering leads at world-class OEMs to evolve our collective supply strategy. In our individual capacities, each of us are determined to do what we can to reliably and consistently provide access to these critical medical devices and supplies.
Following are five of the most important lessons we’ve learned, along with proactive steps suppliers and manufacturers can take to facilitate seamless production in a disrupted supply chain.
1. Consider the Full Range of Potential Delays
The manufacturing sector already was struggling to find enough skilled workers pre-pandemic, and the issue isn’t going away. In fact, staffing shortages across all sectors have been well documented. Specific to the supply chain, however, during the pandemic, the demand for some medical markets decreased as hospital systems and clinics narrowed their focus to essential medical procedures. Meanwhile, suppliers faced staffing shortages due to the pandemic (illnesses, need for childcare, etc.,) and as a result of demand decreases. Unfortunately, now as clinical services are expanding again, suppliers are finding it difficult to rehire and ramp back up. What this means for those of us doing business with suppliers is that they sometimes simply don’t have the human resources to move as quickly to take and fulfill orders as they did pre-pandemic.
Compounding this challenge are shortages of certain medical-grade resins used to make medical plastic products and accessories. This includes the plastic used to make the little clear tamperproof labels used on packaging. As noted above, this means that even if all the materials to produce a medical device or component are available, the end package can’t be finished for delivery. Even in cases where a similar medical-grade plastic is available, these cannot necessarily be swapped out easily. For a new project that’s going through a qualification, this requires a biocompatibility evaluation and material equivalency evaluation. This can sometimes be done within an expedited six to eight weeks, which admittedly in some cases is faster than a long-lead resin’s projected arrival, e.g., 25–30 weeks. For an already approved product, however, the timelines can be longer due to the need to involve the FDA in the change approval process. In either case, however, the product will be delayed.
Even in cases where a supplier in another country can provide the needed material, border patrol and regulatory staffing shortages due to the pandemic creates logjams and delays. For reference, it used to take about a day to get a product from Canada through customs and FDA approval for product manufacturing in the United States. Now it can take up to four weeks. Plus, once you get the go-ahead, delivery fleet and driver shortages can cause further delay.
These are just a few examples of seemingly small factors that pre-pandemic no one really had to worry about, but now may cause significant delays. It goes to quickly understanding where the bottlenecks might be and making sure you’re allocating enough time to take in those surprises and switch gears to other options.
2. Find More Time

In general, there continues to be a shortage of raw materials and extensions in the raw material lead times, especially those materials most commonly used, like medical-grade silicones, various grades of thermoplastic resins, medical packaging (including thermoformed trays and Tyvek lids), metal bar stock, and some specialty packaging labels. It’s gone from predictable to unpredictable across the whole supply chain. Before the pandemic, typically a supplier could fulfill an order within two weeks for implantable-grade plastics, metals, and exotic materials. However, it can now take eight weeks or more, depending on the specific material.
In addition, U.S. legislation dictates how suppliers should prioritize purchase orders for raw materials. In normal times, if shortages exist, priority goes to the military and defense industry. During the pandemic and currently, priority goes to medical supplies that are used to prevent, test, and treat COVID-19, then military and defense, and then everyone else. While these prioritization guidelines make sense, especially in times of scarcity, they do add another layer to the boundaries of supply and demand. In the early months of the pandemic, the raw material supply shortages impacted everything from the production of medical devices to clinical gowns and personal protective equipment. While we have seen some recovery since the darkest supply chain hours of the pandemic, many raw materials continue to be difficult to secure in a timely manner.
On top of this, because of the previously mentioned staffing shortages and potential delays, suppliers are focusing their efforts on producing the most needed raw materials. This is all to say, manufacturers and OEMs need to allow for extra-long lead times and, where possible, develop new products using common raw materials rather than specialty ones.
3. Use Your Lots
Traditionally, suppliers, manufacturers, and OEMs each aimed to balance inventory needs with demand. Logistically and financially, the goal is to have enough in stock to meet demands, but not so much as to waste space or strain finances. However, looking ahead, to stay ahead of supply chain issues, suppliers, manufacturers, and OEMs may need to carry more inventory than in the past until the overall lead times come back to pre-pandemic levels. To be clear though, it needs to be a carefully calculated balance, as “hoarding” is not a go-to option. Outside of the prioritized markets mentioned above, suppliers limit the amount of any one raw material an individual entity can purchase within designated time frames. This means manufacturers and OEMs need to find the sweet spot of having enough on hand to fulfill needs over the longer term, within budget and space constraints.
4. Cross Crafts and Communicate
Pre-pandemic, most of us had discrete roles: OEMs innovated products, manufacturers produced, and suppliers supplied. So, for example, once contracts were signed, there wasn’t much need for interaction between an OEM’s procurement manager and the manufacturer or supplier. Now, however, especially in the beginning of the pandemic, many more (unplanned) hours are spent troubleshooting how to make raw material ends meet and project managing product programs. This unforeseen reality adds time management and budget constraints to all sides. To get ahead of it, stakeholders have adjusted their job descriptions and added “effective communication” to their modus operandi. This shift likely will need to continue — or be initiated, if not already in motion — with parties ready to even overcommunicate on estimated and confirmed delivery dates.
Suppliers should get ahead of any delays by letting manufacturers know as far in advance as possible. Manufacturers should alert their OEM customers of any delays as far in advance as possible. And OEMs should build contingency timing into their timelines and their budgets to accommodate unforeseen or unknowable circumstances that arise. They should also share, to the best of their ability, long-range forecasts for product development needs, so suppliers and manufacturers can anticipate resource needs. The more each party communicates, the farther ahead of delays everyone can be to plan for and implement contingencies. For example, in many cases, OEMs with different product lines buy from multiple suppliers, so they may be able to shift resources based on their pipeline priorities. Likewise, manufacturers may be able to shift gears to find another way to fulfill a need, such as by cleaning and reusing approved packaging trays to compensate for a shortage of new ones.
5. Keep the Playbook in Play
In real terms, medical devices, components, and other supplies help enhance and save lives. Continued vigilance in communication and relationship-building will be in everybody’s best interest. While arguably the once-increasingly transactional nature of “supply and demand” in which the lowest price/fastest delivery won, the impaired pandemic-era supply chain also showed us that you cannot put a price on the value of grit and integrity. Through the chaos, each stakeholder gained a stronger, clearer view into how to work together and help solve one another’s pain points to mutual benefit.
The significant upsets to the global supply chain generally take three to six months to recover from. At the beginning of 2022, the supply chain had begun to level off, with lead times for raw materials beginning to normalize. Then the Omicron variant hit, and it tanked again. Understanding other variants and disruptions could come at any moment, we can learn from the recent supply chain chaos to be better prepared and more collaborative. Among suppliers, manufacturers, and OEMs, each plays an important role in making sure there’s as much access to these technologies and tools as possible.
This article was written by Mike Kaiser, Vice President of Business Operations at Donatelle, New Brighton, MN. For more information, visit here .
Overview
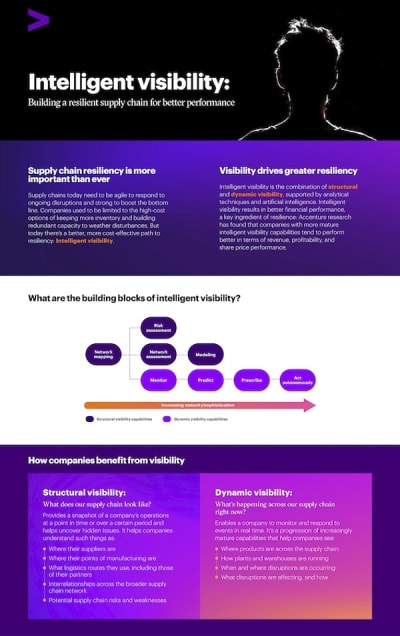
The document discusses the concept of "intelligent visibility" as a critical component for enhancing supply chain resiliency in today's dynamic business environment. It emphasizes that supply chains must be agile and robust to effectively respond to ongoing disruptions while also improving financial performance. Traditional methods of maintaining resiliency, such as holding excess inventory or building redundant capacities, are being replaced by more cost-effective strategies centered around intelligent visibility.
Intelligent visibility is defined as the integration of structural and dynamic visibility, supported by analytical techniques and artificial intelligence. Structural visibility provides a snapshot of a company's operations, revealing essential information such as supplier locations, manufacturing points, logistics routes, and potential risks within the supply chain. This foundational understanding helps companies identify hidden issues and monitor the performance of plants and warehouses.
Dynamic visibility, on the other hand, focuses on real-time monitoring of supply chain events, allowing companies to respond promptly to disruptions. The document highlights that while many companies have achieved a high level of structural visibility maturity, their dynamic visibility capabilities, particularly advanced ones, are often lacking. For instance, while 97% of companies monitor data, only 40% use it prescriptively, and a mere 3% have implemented autonomous execution.
The document outlines four key takeaways regarding the importance of visibility for supply chain resiliency. It suggests that while full visibility may not always be feasible, having a solid foundation of structural visibility is essential for all companies. The goal should be to advance towards more sophisticated dynamic visibility capabilities, which can significantly enhance operational and financial performance.
In conclusion, the document advocates for supply chain executives to prioritize building intelligent visibility capabilities. By doing so, companies can achieve greater transparency, enabling informed decision-making and better preparedness for disruptions. The research indicates that companies with mature intelligent visibility capabilities tend to outperform their peers in terms of revenue, profitability, and share price performance, underscoring the importance of investing in visibility for long-term success.