Starting on Dec. 31, 2012, the Patient Protection and Affordable Care Act’s (ACA’s) Medical Device Excise Tax began to be applied to the sales of all taxable medical devices in the US. Now, almost two years later, the 2.3 percent tax has ruffled investors and stunted new inventions. Industry experts, nonetheless, continue to remain bullish about the future of the medical device innovation sector.
The ‘Hidden’ Tax

When the Affordable Care Act, otherwise known as Obamacare, began rolling out on Jan. 1, 2013, a hidden tax also went into effect—a 2.3 percent medical device excise tax. This provision quickly became a controversial issue. Legislators who support the tax say it’s a justifiable way for a very profitable industry to help finance the ACA, at an estimated $29 billion over a decade, according to the Internal Revenue Service. Industry leaders, on the other hand, say the tax on sales can, and will, harm startups by delaying profitability and forcing cuts on research and development spending, which powers future product development.
For many investors, the excise tax feels like the last straw in a recent onslaught of damaging decisions. “It’s a perfect storm,” said Guy Nohra, the managing director at Alta Partners, a venture capital firm, in EY’s medical technology report. “There are regulatory challenges with the [U.S. Food and Drug Administration], there are reimbursement challenges, there is a lack of available venture capital, corporate buyers are mostly missing in action, and the capital markets have disappeared.”
The medical excise tax is imposed on clinical medical devices, such as CAT scan machines, defibrillators, and other devices sold directly to hospitals and other healthcare providers. (See Figure 1) The tax does not apply to consumer medical devices, such as hearing aids, eyeglasses, and contact lenses, which are purchased directly by a consumer in a store. The IRS defines a clinical medical device as a product “listed as a device with the FDA under section 510(j) of the Federal Food, Drug, and Cosmetic Act.”
Once enacted, the excise tax quickly became a point of contention among medical device startups, investment firms, and fiscally conservative politicians. On April 4, 2013, the Senate Finance Committee passed a tax reform bill that many legislators and industry experts thought would address the hidden tax. When the “tax extenders package” was passed, it covered 50 provisions worth $85.3 billion—but it very publically left the excise tax unrepealed. As a result, the provision received yet another bout of negative attention.
The Changing Marketplace
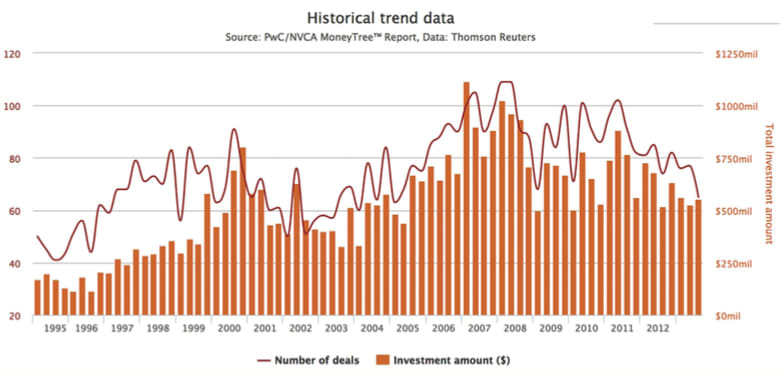
Since the tax implementation, the investment market has noticeably dipped. Total venture capital (VC) funding of medical device startups fell about 19 percent in 2013 over the previous year to $2.1 billion in 2013, from $2.6 billion in 2012, according to a MoneyTree Report by PricewaterhouseCoopers and the National Venture Capital Association. At its 10-year peak in 2007, medical device and equipment funding reached nearly $4 trillion.
“Financing has become increasingly scarce for small companies,” said EY in its Pulse of the Industry: Medical Technology Report 2013. “Slowing growth has resulted in ‘lost’ revenues of $131 billion and ‘lost’ research and development of $12 billion between 2008 and 2012.”
The recent drop in investment funds is especially harmful to medical device startups that are still a couple of years away from getting their products on the market. Potential acquirers, for example, may be less likely to fund certain devices, as the pressure on sales and profits builds through a raised price point used to pay for the hidden tax.
In a 2013 survey by the Advanced Medical Technology Association (AdvaMed), a trade association that benefits medical technology firms, 75 percent of its survey respondents, which were composed of the association’s partner companies, said they had “deferred or cancelled capital investments,” “reduced investment in start-up companies,” and “found it more difficult to raise capital among startup companies,” as a direct result of the excise tax.
While the biggest problem startups face is acquiring a strong financial backing for their products, medical device entrepreneurs, on a basic level, are finding it difficult to understand the provision — exactly which devices are subject to the excise tax?
As referenced earlier, some products, such as devices sold directly to consumers at drug stores, aren’t subject to the tax. But for many manufacturers, this distinction is a confusing gray area. Mobile health apps and software that have been cleared through the FDA, for instance, go through the same processes as a medical device, yet the government has not determined whether or not they are subject to the excise tax, creating an air of uncertainty within the community.
In addition to the mounting confusion among US startups and investment firms, the volatile tax fluctuations are causing some US-based multinational companies, such as medical device developer and marketer Boston Scientific, to make sizeable investments abroad while simultaneously announcing workforce reductions at home. Because the tax is added to the total sales cost in the US, it makes no discernible difference whether the medical device is manufactured in the US or abroad, yet companies are disenfranchised to grow businesses in the US nonetheless.
Almost 10 percent of AdvaMed’s survey respondents said they had relocated manufacturing outside the US or expanded manufacturing abroad rather than expand in the US. While increased investments overseas may not be a direct result of the excise tax, the implication speaks volumes about the current state US marketplace for startup funding and innovation.
The Future of Medical Device Startup Funding
Securing a patent, navigating tax law, and obtaining funding are just a few examples of the litany of challenging steps a medical device startup must take when trying to make their ideas come to life. Now, with a shrinking investment pool, complicated tax regulations, and companies pumping money into businesses outside the US, the future of American medical inventions is unclear.
One trend, however, is crystal clear — investors are still looking for ways to fund novel medical inventions. In the first quarter of 2014, medical devices and equipment received about $600 billion in investment funds, according to a MoneyTree Report by PricewaterhouseCoopers and the National Venture Capital Association. Of that amount, VC firms contributed nearly $1 billion, which is slightly ahead of 2013’s pace, while still remaining down from 2011 and 2012 levels, according to the report. (See Figure 2)
More than ever, medical device startups are exploring new avenues for navigating the dearth of VC funding and the seemingly endless list of steps that need to be achieved before a product can successfully go to market. For example, new and evolving business models such as healthcare innovation marketplace Edison Nation Medical or healthcare incubator Healthbox are able to leverage their expansive networks and resources to aid inventors in bringing their disruptive medical inventions to life.
“Within the medical technology industry, it is easy to bemoan the apparent pressure this ‘new’ healthcare environment places on innovation,” said Omar Ishrak, chairman and CEO of Medtronic, in EY’s medical technology report. “However, this very reaction suggests that innovation within our industry is limited to technology alone. Instead of defining innovation as inventions and iterations, we must focus on the opportunity to redefine innovation across our industry to include bold new business models that deliver clear clinical and economic value to health care systems.”
While traditional pathways to funding innovation feel like a thing of the past, it is undoubtedly an exciting time to have a novel idea in the medical community. Despite a dip in funding since the excise tax was enacted, there remain consistent signs of investment firms’, healthcare marketplaces’, and incubators’ growing interest in making deals with medical device startups.
“At a time when access to venture capital has become more difficult and IPOs have diminished as a viable financing option,” said EY representatives in their 2013 medical technology report. “The outlook for deals remains bullish.”
This article was written by Bobby Grajewski, President, Edison Nation Medical, Charlotte, NC. For more information, Click Here .



