
Sterilization represents a critical step in ensuring the safety and biocompatibility of medical devices. The process necessitates early consideration during the design of new devices due to the potential incompatibility of many materials with prevalent sterilization techniques. The appearance, material performance, and biocompatibility of medical devices can be impacted by various sterilization methods. Predominantly, medical devices undergo sterilization through ethylene oxide (EtO) treatment or exposure to ionizing radiation, such as gamma rays or electron-beam (e-beam) irradiation. EtO sterilization and gamma sterilization are the most prevalent; however, emerging methods utilizing x-rays, microwaves, and e-beam are growing. While steam sterilization (autoclaving) is an option, particularly in hospital or clinical settings where reusable tools and devices are employed, it is unsuitable for sterilizing single-use plastics, dressings, electronic components, or intricate designs.
Traditionally, silicone pressure-sensitive adhesives (PSAs), which are the most skin-friendly adhesives for attaching medical devices to the skin, could only be sterilized with EtO. This article examines a new silicone PSA capable of enduring sterilizing doses of gamma radiation. This recent advancement enables numerous new applications for silicone PSAs in medical devices that were previously unattainable.
Limiting Use of EtO
While EtO sterilization is widely used in the industry, it is not without concerns. It is highly effective in sterilizing medical devices, but it is also highly toxic and carcinogenic. In March 2024, the United States Environmental Protection Agency (EPA) issued a final ruling limiting the use and release of EtO gas by commercial sterilization operations to 90 percent. 1 This ruling was influenced by studies linking breathing problems and proximity to EtO sterilization facilities.
Strict regulations prohibit the construction of sterilization facilities near schools due to environmental cancer rates associated with their operations. As regulations on EtO use continue to be implemented, the cost of sterilization using EtO is expected to increase significantly.
The EtO sterilization process involves filling the chamber with EtO gas, maintaining it for a certain period, evacuating the gas, and allowing time for degassing. It can take days or up to a week to complete, depending on the device’s complexity. EtO sterilization is used when no other techniques are compatible with the materials in the medical device, making it a significant consideration in the supply chain of medical devices due to its time-consuming nature and regulatory scrutiny.
Sterilization techniques that employ ionizing radiation, such as gamma and e-beam, are preferred if the medical device is composed of materials that can withstand such high energy radiation. Exposure to gamma radiation is a favored method for sterilization due to its ability to deeply penetrate complex, densely packed containers full of medical devices/components.
Gamma sterilization facilities can sterilize medical devices much more quickly than EtO facilities. In a gamma sterilization facility, a container of items is placed onto a conveyor belt and allowed to traverse a chamber that has that has a gamma source centrally located within in it. After a few hours of passing through the chamber, the container holding the medical devices comes out the other end. While passing through the chamber, the contents of the container absorb enough energy from the radiation to kill any germs that may be present. This process allows sterilization without significant temperature elevation and ensures that there is no toxic residual chemical vapor contamination.
Silicones: Designed to Adhere to Skin
For medical devices that adhere to skin, it’s important to use the most suitable PSAs for each application. The most used, skin-compatible PSAs in the industry are acrylates, hydrocolloids, and silicones. When comparing these options, each has its pros and cons, but silicones are the best adhesives for skin-friendliness.
The chemical nature of silicone provides inherent breathability that is unmatched by other synthetic polymers. Therefore, silicone PSAs allow skin to breathe, and maceration (i.e., weakened skin due to oversaturation with moisture) is not a problem with silicone PSAs. Silicone PSAs designed for use on skin are much gentler and do not cause damage or leave residue when removed. Silicones’ perfect suitability as skin adhesives is further supported by their nontoxic and hypoallergenic nature.
Wacker provides a wide range of two-component room-temperature-curing (RTV-2) silicone skin adhesive products. They are easy to use — simply mix the two liquid components in a 1:1 ratio and apply the mixture to the desired substrate. These adhesives can be applied using various methods, depending on the manufacturing scale. They can be brushed on, poured onto surfaces, or coated using common coating techniques such as knife over roll or slot die on roll-to-roll coating lines. While these adhesives cure at room temperature, curing can be accelerated at higher temperatures.
These adhesives are ideal for creating skin-friendly products due to silicone’s clean chemistry, which produces no toxic or hazardous byproducts and doesn’t require the use of flammable solvents. Sold under the SILPURAN® brand, a variety of silicone skin adhesives offer a range of peel, hardness, and tack properties for use in different skin fixation applications. These adhesives are ideal for dressings used in advanced wound care. They are suitable for patches that minimize the appearance of scars and wrinkles and provide gentle yet secure adhesion for reusable wearable devices and appliances.
Gamma-Sterilizable Silicone Adhesives
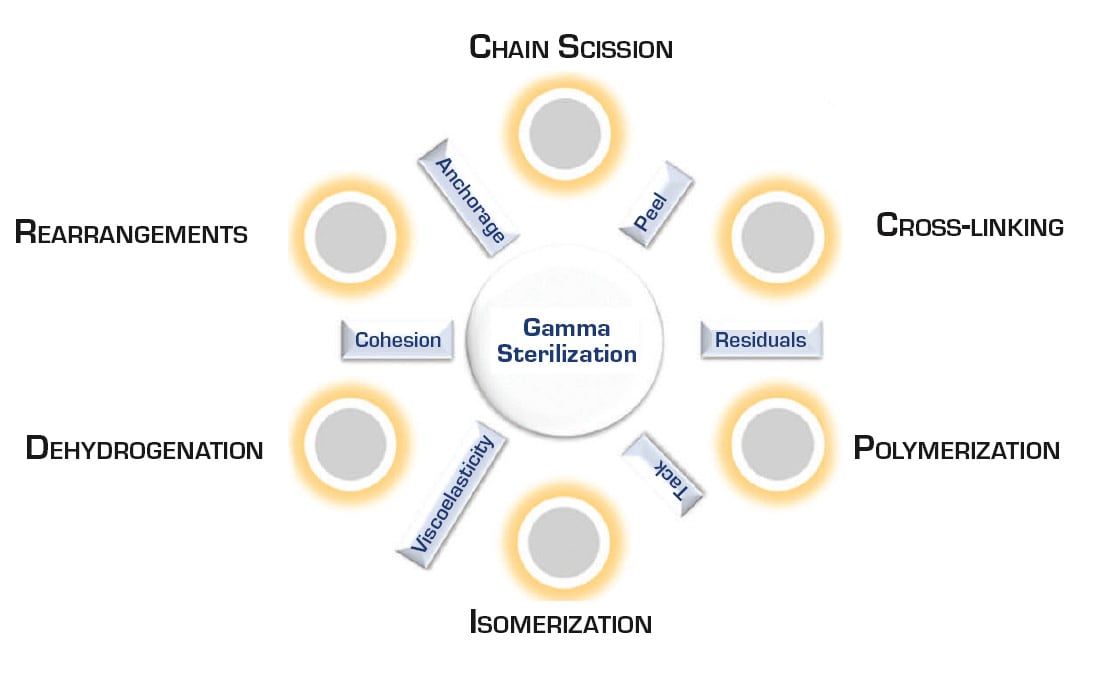
Traditional silicone PSAs are perfect for skin but not quite perfect for use in all medical device applications. Silicone polymers and elastomers are not stable to ionizing radiation, like gamma. Silicone generally is not the least stable material when compared to other materials for stability under gamma radiation at sterilization doses; however, silicone PSAs are highly affected. The potential reactions from gamma exposure are summarized in Figure 1.
Gamma radiation affects silicone PSAs, and all PSAs in general, because PSAs are dependent on maintaining a certain viscoelastic profile to stay sticky and tacky. In other words, if the silicone flows too much, it is and gooey mess and, if the silicone becomes too hard, it becomes a non-tacky rubber. Gamma radiation makes traditional silicone PSAs harder and turns them into a rubber. The only sterilization technique that works for traditional RTV-2 silicone skin adhesives is EtO treatment.

Wacker’s Healthcare Center of Excellence team has created an innovative silicone PSA that can endure sterilizing doses of gamma radiation and retain its adhesive properties. Figure 2 shows a comparison of tack measurements before and after gamma sterilization of this new PSA as well as other traditional silicone PSAs. The new adhesive utilizes patented technology that stabilizes the adhesive so that it does not undergo significant changes in its adhesive properties when exposed to gamma radiation up to 25 kGy.
The two Wacker adhesives, referred to as Adhesive 1 and Adhesive 2 in Figure 2, are gamma stable. The physical properties of these adhesives were evaluated after exposure to a 25 kGy dose of gamma radiation and compared with other Wacker silicone adhesives, specifically those marketed under the SILPURAN brand. All adhesives were coated at 15 mil wet-film thickness on Mylar® substrates. The adhesives’ physical properties, including probe tack and 180° peel, were examined before and after gamma exposure at 25 kGy.
The adhesive coatings were cut into 25-mm-wide strips and attached to the sample holder. Peak tack was measured using a 7-mm stainless steel flat probe. Figure 2 illustrates the changes in tack before and after exposure to gamma radiation.
The adhesive strips, measuring 25 × 300 mm, were applied to stainless steel panels using a roller for even adhesion. After resting for 30 min, a peel adhesion test was performed at a 180° angle and reported in N/in. Figure 2 shows the changes in peel upon radiation. The results obtained before and after gamma irradiation revealed a clear difference in the performance of the adhesives.
SILPURAN adhesives exhibited a significant decrease in both tack and peel strength, indicating that these materials are susceptible to degradation or cross-linking when exposed to gamma irradiation. In contrast, the two new adhesives maintained their adhesive performance with minimal changes in comparison to traditional soft silicone adhesive gels.
Summary
When determining the method for sterilizing a medical device, the selection of technique relies on the materials used and the complexity or shape of the device. In today’s medical device market, the two primary techniques are treatment with EtO or exposure to gamma radiation, each having its own advantages and disadvantages. Traditionally, silicone PSAs, which are the most skin-friendly adhesives for attaching medical devices to the skin, could only be sterilized with EtO. However, a new silicone PSA is capable of enduring sterilizing doses of gamma radiation. This recent advancement enables numerous new applications for silicone PSAs in medical devices that were previously unattainable.
Reference
- “Final Amendments to Strengthen Air Toxics Standards for Ethylene Oxide Commercial Sterilizers,” U.S. Environmental Protection Agency, March 2024.
This article was written by Dr. Shabnam Pordel, R&D Scientist; Steven Mankoci, Technical Manager; and Kyle Gaines, Healthcare Solutions Marketing Manager; Wacker Chemical Corporation, Ann Arbor, MI. For more information, visit here .



