Manufacturing electronic medical devices and the complex printed circuit boards (PCBs) within them demands stringent quality control measures to ensure the highest level of performance, reliability, and safety. Process validation is a critical step in the production cycle to verify that manufacturing processes consistently produce products of the highest quality.
Parts cleaning with cleanroom compatibility plays an important role in achieving successful process validation by removing contaminants that can compromise the functionality and integrity of these intricate components. For this reason, it is critical to consider the cleaning process at the early planning and design stages. Although often overlooked, cleaning techniques will help to successfully attain process validation to pass quality control and ensure product longevity.
Why Cleaning Is Important for Process Validation
Electronic medical devices often have intricate designs and delicate PCBs that are sensitive to contamination. Any contamination like residual oils, flux residues, or other particulates can adversely affect these devices’ consistency and functionality and impact the validation process. Therefore, cleaning is essential to ensure that they meet the required quality standards and regulatory compliance.
When cleaning electronic medical devices, there are five reasons why process validation is important:
- Compliance with Regulations: Regulatory bodies, such as the Food and Drug Administration (FDA) and International Standards Organization (ISO), require medical device manufacturers to validate their cleaning processes. Validation demonstrates compliance with standards and ensures that the devices meet stringent quality and safety requirements.
- Patient Safety: The electronic components must deliver reliable and consistent performance, particularly as they are often used in life-sustaining medical devices. Therefore, cleaning procedures must be implemented during the manufacture of these multifaceted assemblies to guarantee their reliability and meet process validation requirements.
- Reliability: Validation establishes that the cleaning process constantly achieves the desired level of cleanliness. It verifies that the process parameters, such as cleaning agents, temperature, time, and equipment, are properly controlled and optimized to consistently achieve the required level of cleanliness for the device throughout the manufacturing process.
- Quality Assurance: Cleaning helps manufacturers establish protocols that maintain the devices’ performance and prevent any adverse effects resulting from residues or contaminants.
- Documentation and Traceability: Process validation requires comprehensive documentation of the cleaning process, including standard operating procedures (SOPs), cleaning methods, parameters, and validation results. It enables manufacturers to track and evaluate the effectiveness of the cleaning process over time.
Challenges in Cleaning Complex Medical Devices

Cleaning electronic medical devices poses specific challenges due to their intricate designs, tight spaces, and multifaceted PCB designs that are difficult to clean and dry. Traditional cleaning methods may struggle to remove contaminants from these hard-to-reach areas effectively. Furthermore, the smaller size of modern medical devices adds to the difficulty of thorough cleaning and can trap contaminants.
The cleaning process must be carefully designed to ensure that it meets the cleanliness standards required for medical devices. It is important to select a cleaning process that effectively removes contaminants without causing damage to the delicate components or surfaces that are often used within medical devices. Additionally, the chosen cleaning method must be compatible with established procedures and offer consistency and ease of use to meet process validation requirements.
The Benefits of Vapor Degreasing
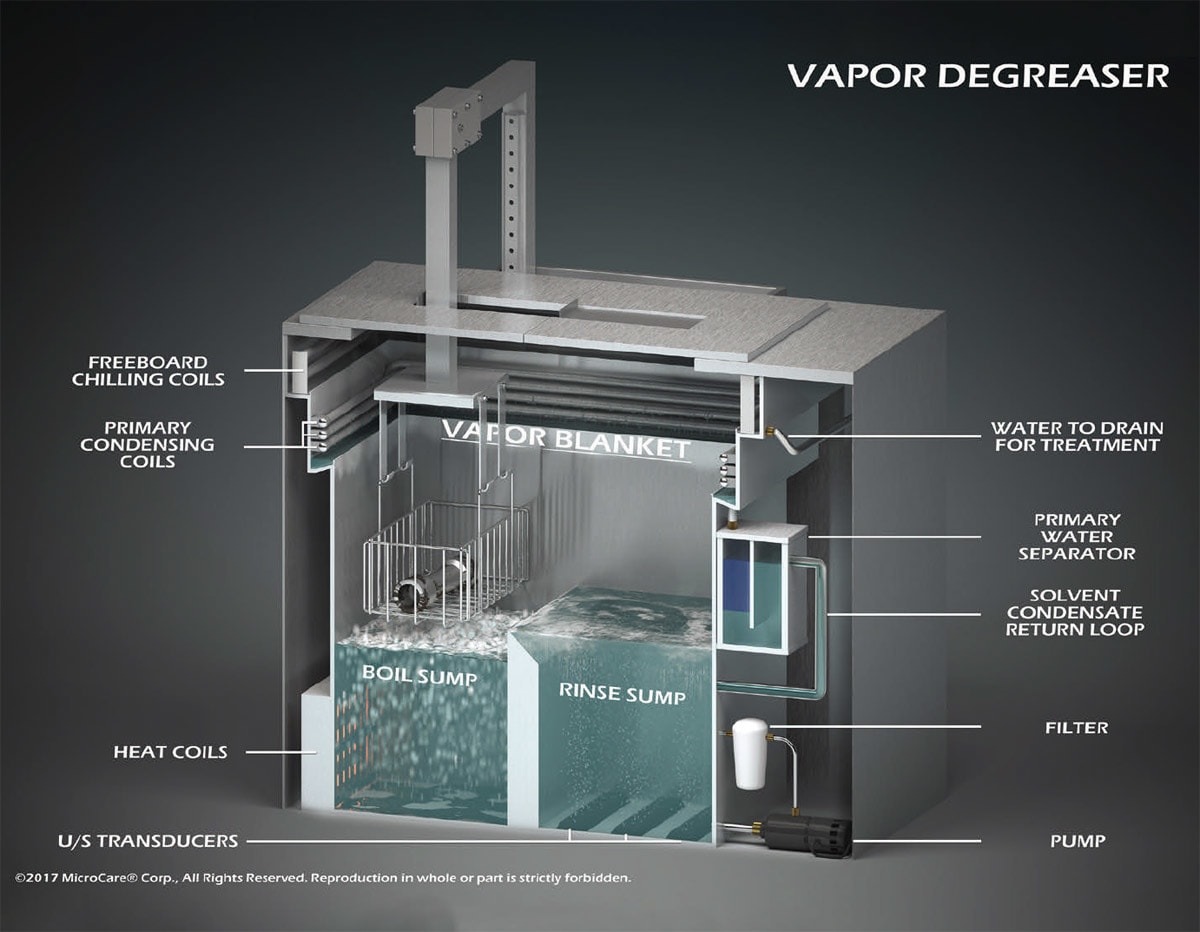
Vapor degreasing is a well-engineered cleaning process that offers several benefits when cleaning electronic medical devices. In a vapor degreaser, the cleaning process occurs within a closed-loop system comprising two chambers: the boil sump and the rinse sump. The boil sump contains a specially formulated low-boiling, non-flammable cleaning fluid. Parts are immersed and cleaned inside the heated fluid, then transferred to the rinse sump for a final rinse and dry. This process ensures that the components emerge clean, dry, spot-free, and immediately ready for the next steps in the manufacturing process, such as assembly, finishing, sterilization, or final packaging.
One significant advantage of vapor degreasing is its effectiveness in cleaning delicate medical device parts without causing damage. Modern cleaning fluids used in vapor degreasers are specifically engineered with low boiling temperatures, low surface tensions, and low viscosities. This allows the cleaning fluid to penetrate tight spaces, dissolve contaminants, and evaporate without leaving residue behind. Modern cleaning fluids also combine higher density and lower viscosity, which aids in lifting and floating particulate from the parts, ensuring thorough cleaning.
In addition to its cleaning effectiveness, vapor degreasing offers other benefits that simplify the process validation for medical devices. The consistency of the cleaning process is a key advantage. Once established and tested, the cleaning fluid inside the vapor degreaser remains chemically stable for thousands of uses, eliminating the need for frequent maintenance and daily monitoring. This stability ensures reliable cleaning results that comply with process validation specifications.
Because vapor degreasers come in various sizes, they can accommodate different batch sizes and part geometries. Whether small batch work or mass-produced parts, the cleaning results remain stable and repeatable. This eliminates the need for equipment additions or updates when introducing new products, even if they are of different sizes or made from different materials. The consistency and auditable nature of the vapor degreasing process simplify validation requirements, as a complete cleaning record can be maintained to demonstrate adherence to defined procedures and instructions.
Importantly, vapor degreasing offers reliability and repeatability, making the documentation simple. The systematic nature of the process facilitates easy engineering, auditing, and streamlined recordkeeping. It allows for comprehensive tracking of the cleaning history of a batch, demonstrating adherence to defined procedures and instructions and validating the expected outcomes.
If parts are manufactured within a cleanroom environment, vapor degreasing helps to maintain the necessary environmental conditions, including temperature and humidity. No dust, fumes, heat, or moisture is produced, therefore eliminating the need for specialized blowers, fans, or climate control systems to maintain air quality, temperature, and humidity.
Finally, modern cleaning fluids used in vapor degreasing are hostile to pyrogens and will minimize the bioburden risk. Because PCBs come out of the vapor degreaser clean, dry, spot-free, and cool enough for immediate coating or packaging, the possibility of bioburden contamination is reduced considerably as any moisture is removed making it a convenient means to validate a cleanroom-compatible cleaning process.
Reliability and Validation
Cleaning plays a crucial role in process validation for manufacturing electronic medical devices. It ensures the highest performance, reliability, and safety levels. Cleaning removes contaminants that can compromise device functionality and longevity, making it an important process to consider early in the planning and design stages.
Vapor degreasing, using modern cleaning fluids, effectively cleans small complex parts. It simplifies the validation process with its repeatability and consistency to ensure safety and regulatory quality standards are always met.
This article was written by Elizabeth Norwood, Senior Chemist at MicroCare, LLC, New Britain, CT. Norwood has been in the industry more than 25 years and holds a BS in Chemistry from the University of St. Joseph. She researches, develops, and tests cleaning-related products. She currently has one patent issued and two pending for her work. For more information, e-mail



