
Pulsed-field ablation (PFA), also known as irreversible electroporation (IRE), is a rapidly emerging technology for tissue ablation that is seeing robust excitement for use in cardiac electrophysiology. Major medical device manufacturers are making billion-dollar investments in this new technology, and the clinical community is expecting a rapid shift from existing tools to PFA within the coming years. With global volume approaching 1 million procedures annually, growing at ~15 percent year over year, the impacts to the medical device industry, clinicians, and patients are certain to be significant.
This article discusses the electrophysiology clinical applications for PFA, the rationale for pursuing PFA over other technologies, other clinical areas that are likely to be impacted by this technology in the future, and the challenges with designing PFA systems.
Clinical Application: Arrhythmia Correction
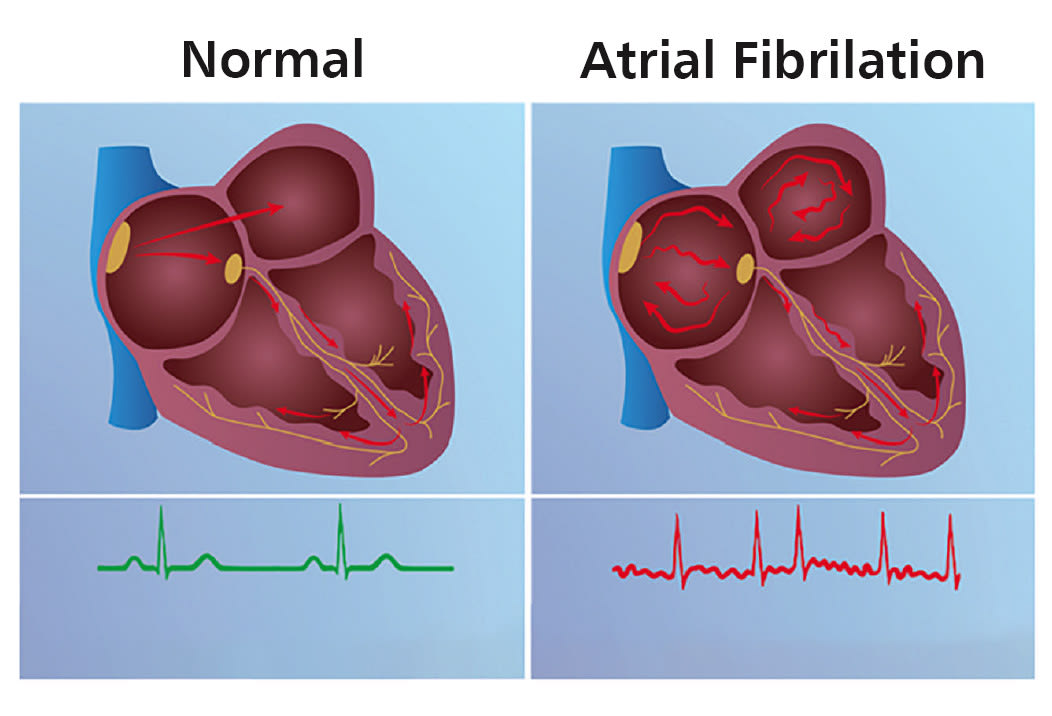
One in 10 adults over age 65 suffers from atrial fibrillation, a type of heart arrhythmia in which errant conduction pathways within the heart’s signaling system allow certain chamber(s) of the heart to beat erratically. Figure 1 illustrates the general condition. These erratic beats lead to inefficient pumping, pooling of blood, and the potential for stagnant blood to form clots. If these clots leave the heart, they can cause a stroke. Thus, correction of the arrhythmia is often undertaken as an elective, prophylactic procedure.
Today, this procedure involves identifying then ablating (killing) the tissue responsible for the errant conduction pathways. Catheters are advanced into the heart under fluoroscopic guidance, electrical mapping systems are used to identify the areas to target, and radio-frequency (RF) or cryogenic systems are used to create lesions to electrically disconnect the erroneous connections within the heart’s musculature, restoring a normal heart rhythm.
When Problems Arise
While very effective, traditional thermal ablation methods (like radio-frequency and cryogenic) risk incidental damage to important adjacent tissues like the esophagus or phrenic nerve. Serious damage to the esophagus, should it occur, is a complication that is likely fatal; such an outcome from an elective procedure is an unacceptable risk.
The risk of collateral tissue damage is inherent to any thermal-based system, as almost all tissue in the body (with the notable exceptions of fat and bones) have very similar thermal conductivities (ability to transfer heat) and thermal capacities (ability to absorb heat) — the heat (or cooling, in the case of cryogenic) has no way to differentiate the target tissue from sensitive adjacent tissue. The clinician is tasked with attempting to wield the thermal energy in a manner that is therapeutic without being injurious — a tall order in a small space.
Electric Fields to the Rescue
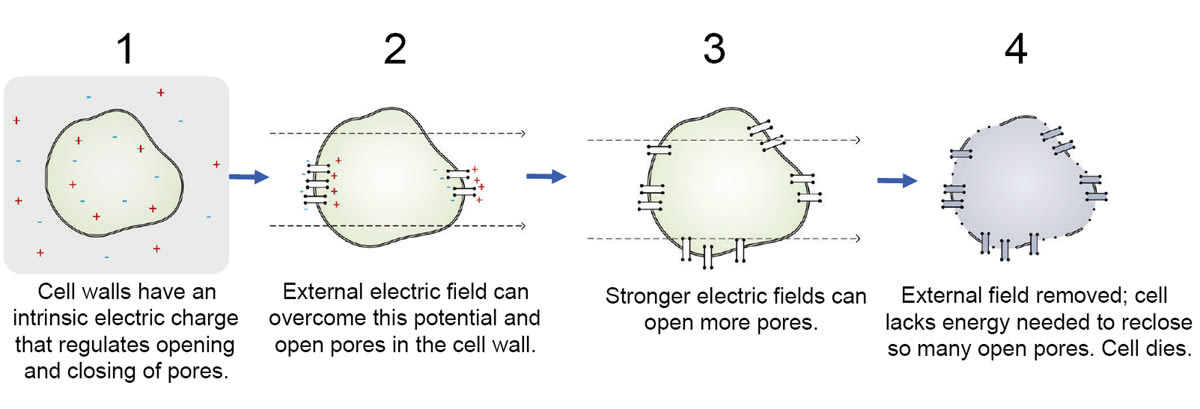
Unlike thermal modalities (which rely on heat), PFA relies on the use of the electric fields. Rather than a macro-scale thermal process, it is a micro-scale, cellular-level process. Electric fields can cause the opening of cellular pores, which can become stuck open, depleting the cell of needed energy, and resulting in cell death. Through adjustment of the electric field strength, the number of pores opened can be controlled. While superficially the same (insofar that it involves a source of electric current and a conductive catheter), it is radically different in its mechanism of action. Figure 2 illustrates the PFA mechanism of action at a cellular level.
Most critically, the benefit of electric field-mediated cell death is that different cell types have vastly different susceptibilities to the electric fields generated via PFA-mediated ablation, as illustrated in Figure 3. In the case of cardiac electrophysiology, the types of cells responsible for atrial fibrillation (myocardium) are affected by relatively weak electric fields, while the adjacent tissues of concern (esophageal smooth muscle and nerve) require markedly higher field strengths before they experience any electric field-induced damage.

Practically, this means that a PFA system for cardiac electrophysiology can be designed to supply double the needed electric field strength, while still having a 100 percent safety margin to collateral tissue damage. The clinical benefits of this inherent safety mechanism (and its enormous magnitude) cannot be overstated.
Finally, the patient-to-patient variability encountered by electric field-mediated ablation is small (smaller than in thermalbased ablation), making the procedure more predicable and consistent. In summary: The benefits of PFA in electrophysiology include easy, fast, highly effective procedures, significantly reduced risk of complications due to thermal injury, and consistency and predictability.
The Future of PFA in Electrophysiology
PFA in electrophysiology is still an emerging technology. First-generation products have just recently been introduced to the European market, and one commercial system has recently been FDA approved via the Breakthrough designation pathway. However, results from clinical trials have been incredibly promising, to the point that some commentators expect PFA to almost completely usurp the use of thermal ablation modalities in the electrophysiology market within the next few years.
More likely, many clinicians who are presently trained in (and proficient with) radiofrequency or cryogenic ablation will continue use of these technologies until compelled to switch. Radio-frequency and cryogenic ablations are still highly effective therapies with low incidences of side effects. Experienced clinicians can perform these procedures quickly, effectively, and quite safely.
Much of the excitement around PFA in electrophysiology seems to be a blue sky effect. Specifically, relatively inexperienced users of PFA are demonstrating procedural speeds, efficacies, and rates of complications that are equivalent to extant thermal modalities. Equivalence is not a compelling rationale for a wholesale technological shift in a multi-billion-dollar market; there is clearly some anticipation that clinical experience and further innovation will deliver even greater positive outcomes.
Technologically, there is still significant basic science work remaining to understand and optimize the numerous parameters which affect performance. This lack of understanding has given rise to a “proprietariness” problem: every system must be regarded as completely proprietary, with every attribute of its performance (from catheter geometry to field strength intensity, to pulse timing and duration) completely unique to that specific device for that unique clinical application, which limits opportunities to generalize performance across systems or implementations.
Other Clinical Applications of PFA
Given the robust excitement for PFA in electrophysiology, there has also been emerging interest in applications of PFA in other clinical areas. These are even newer, and not as well understood, but could presumably include any application which can also demonstrate a similar clinical benefit in terms of tissue selectivity, safety, procedural speed, or ease-of-use. Examples of applications where known clinical work is ongoing include:
Prostate cancer and prosthetic hyperplasia.
Pancreatic cancer.
Mucosal remodeling.
Lung cancer.
Diabetic foot ulcers (DFUs).
Skin cancer.
Gastrointestinal cancer.
Many of these applications may theoretically benefit from the advantages of PFA, but the basic science identifying the particular value (e.g., cell-type specific ablation thresholds to avoid collateral tissue damage) are less well-known than in the electrophysiology market. These indications will require significant clinical work to advance PFA technology.
Challenges in PFA System Development
PFA systems are unlike most any other energy-based medical device. They require very fast electrical pulses, delivered at very high voltages and high currents, which strain the capabilities of electronics, control systems, and sensors. Electrical hazards take different forms than known in other devices, and expert guidance is needed to advance development of these systems without encountering pitfalls.
Clinically, significant investigation is ongoing regarding the most optimal catheter designs, electric field prescriptions (waveforms and parameters), and pulse-delivery algorithms. Unlike thermal modalities (where tissue temperature is readily measured and directly correlated to clinical effects), electric field therapy’s efficacy cannot be directly or immediately measured in situ. This gives rise to another challenge, which is that every clinical indication, every PFA system, and every configuration permutation of a given system requires some level of clinical evidence to assess performance and safety. Being a new technology, and with substantial “unknowns” in the basic science, the concept of “substantially equivalent” will be a challenge to define.
Overcoming these development hurdles will require deep knowledge of the technical, regulatory, and clinical challenges, ideally from a partner who has solved these problems before.
Future Outlook
There is no doubt that the tissue selectivity benefits of PFA are likely to be the leading cause of its widespread adoption in cardiac electrophysiology in the very near future. And, as PFA technology matures, it is also likely to provide clinical benefits in the treatment of other diseases and therapies. The flurry of investments, development work, clinical trials, and marketing of this technology will benefit those companies that are most knowledgeable in the space, understand the technology from a first-principles level, execute product development with an appreciation of the unique challenges of this technology, and can navigate the complex interactions between disparate system components.
Developing a PFA Partnership
A design, development, and manufacturing partner to the medical device industry is crucial. Minnetronix Medical, for example, has a specialization in surgical energy devices: devices (such as RF and PFA generators), which deliver electrical energy to tissue. Its ~500 employees (of which 200 are degreed engineers and scientists) solve complex medical device challenges, currently with more than 40 active development projects. The team manufactures more than 25,000 finished medical devices annually, ranging from small wearable sensors to refrigerator-sized equipment consoles. The Minnetronix surgical energy team has developed more than 30 RF and PFA generators for clinical applications, including electrophysiology, oncology, denervation, and other indications across the entire human body.
This article was written by Dan Friedrichs, PhD, who leads development engineering efforts, and Aaron McCabe, PhD, who is technology segment director focused on surgical energy applications at Minnetronix Medical, St. Paul, MN. For more information, e-mail



