Total hip and knee replacements are very common and have an excellent track record of success. Over time, however, they can fail for a variety of reasons like implant wear/loosening, recurrent dislocation or infection. When failure occurs, the physician may recommend a second operation, called a revision surgery, to remove some or all the parts of the original implant and replace them with a new ones.
Revision surgeries are much more complex than the primary surgeries and as a result, planning these surgeries requires a detailed understanding of an individual subject’s anatomy and how it interacts with an implant. To model the latter, 3D imaging such as computed tomography (CT) can be used to create a realistic representation of anatomical structures.
However, taking this image data and extracting relevant information from it is often a time-consuming and predominantly manual task for clinical professionals, involving careful segmentation of key body parts and selecting relevant anatomical landmarks. This significant bottleneck then has an effect on the time needed to plan surgeries and can contribute to unwanted delays. One emerging solution for this challenge is automated segmentation and landmarking using machine learning-based artificial intelligence (AI) to speed up tedious tasks. More generally, AI-based solutions enable significant acceleration of image-to-model workflows in the life sciences and give clinicians and medical device engineers more time for analysis and innovation.1 How does this work, and what kind of cases can it benefit?
Revision Surgery Challenges
According to recent studies, the number of total knee arthroplasties (TKAs) in the United States alone will increase from 719,000 in 2015 to 3.48 million by 2030, with contributing factors including an aging population, increased obesity rates, and the potential of new technology to open up access to procedures at lower costs. Furthermore, total hip arthroplasties (THAs) are predicted to rise from 332,000 to 572,000 over the same time span, creating increased demand from patients and increased pressures on clinicians.2
Subject-specific surgical planning is also now becoming common, with image data taken from CT and magnetic resonance imaging (MRI) making it easier to create 3D models that accurately capture unique anatomies and pathologies. Advantages of this approach include optimization of hip and knee implants to individual patients, reducing the risk of complications and improving comfort. The use of customized implants requires careful presurgical planning using simulation of how medical devices interact with the body, reducing the time spent on design iterations and increasing confidence in decision-making in the run-up to operations.3
However, revision surgeries are still required for hip replacements after ten years in about 4–5 percent of cases, going up to 15 percent after 20 years, while it is expected that the annual number of knee revision procedures will increase at a rate of almost 90 percent from 2020 to 2050.4 As a result, it is important for these procedures to be efficient and designed to prevent recurring problems in both straightforward and more drastic scenarios. This issue is compounded as success rates for revisions are generally lower than initial surgeries due to a weakening of the bone.
Manual 3D Image Segmentation Bottlenecks. When planning a revision surgery, clinical professionals working with MRI and CT data face several bottlenecks to creating a workable solution for an individual patient. Most notably, segmentation and landmarking of the different regions of interest within anatomical data can be a painstakingly manual process. Depending on the technical experience and skill of the user, many hours may be spent just getting to the point of identifying the anatomical structures that are relevant to a surgery.
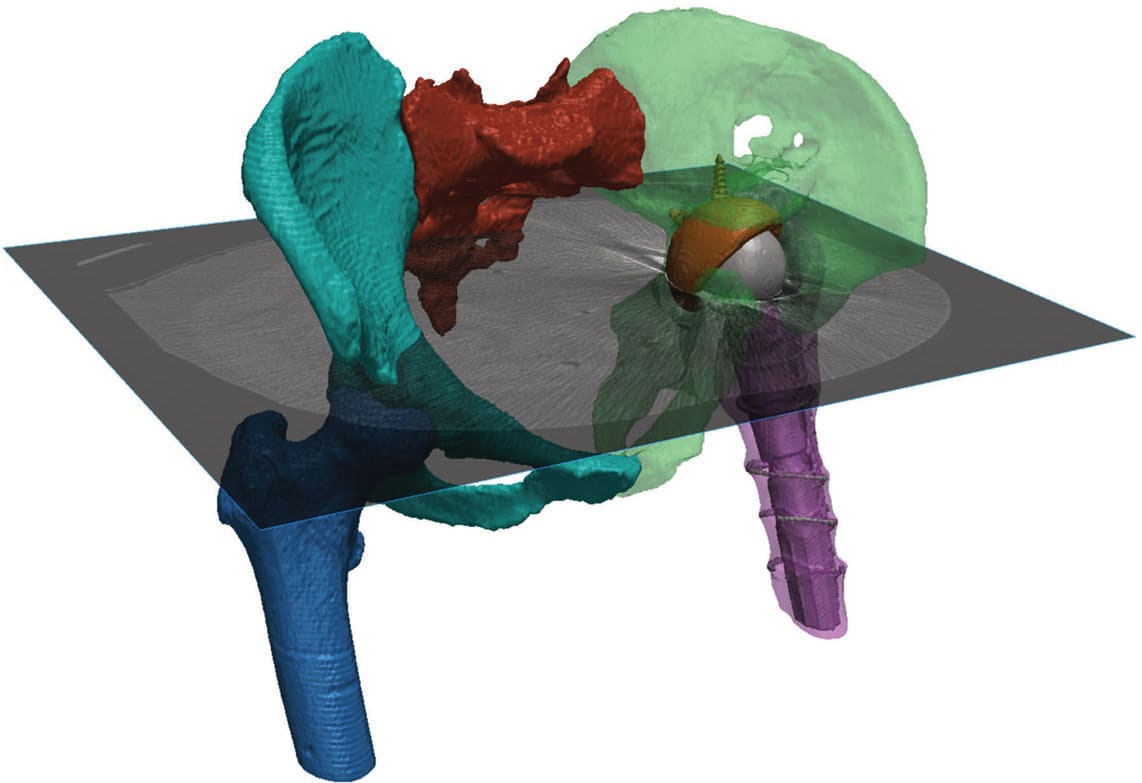
Automated Segmentation for Different Revision Cases. AI is becoming increasingly recognized in the medical field when dealing with these repetitive challenges, for clinical detection and patient-specific surgical planning or design purposes. Synopsys Simpleware™ software is one example of where AI has been applied to a specific problem in manual segmentation, and uses machine learning to automate repetitive steps and landmarking in order to speed up workflows (see Figure 1). Patient datasets and extensive training inputs are used to develop algorithms that can, with a single click, automate a large part of the segmentation on an anatomy, with clinical reviews used to ensure suitability for real-world cases. This technology has been applied through Simpleware AS Ortho, a software module that works specifically with hips, knees, and ankles.
In the case of primary and revision planning, those working with patient data can use automated segmentation to identify different anatomical structures to quickly generate a standard result, including masks and landmarks in both a 2D and 3D output that is ready for further analysis. Each femur, the pelvis, and sacrum can be distinguished, and common landmarks automatically placed. From this point, additional editing tools then enables users to work on specific features and prepare a final model for CAD work, such as positioning an implant, or finite element analysis (FEA)-based simulation. For common workflows, this means a 20–50 times faster rate of segmentation and landmarking, which saves time and cost when scaling medical device R&D through to surgical planning.
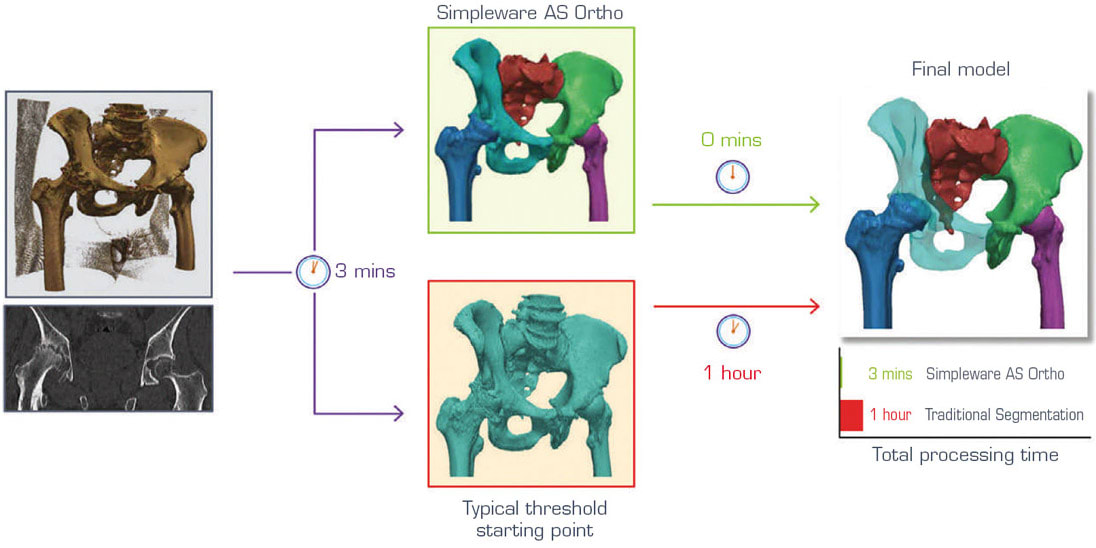
For primary surgical cases involving hips, automated segmentation makes it much easier to work with CT scans to extract the relevant information than typical approaches. While separating the hip and femur in these planning stages is relatively straightforward for users, it can still take an hour or so for the average user to complete with manual and semiautomatic methods such as thresholding, region growing, etc. When using automated segmentation, a final model can be obtained in a few minutes with just one click, as shown in Figure 2.
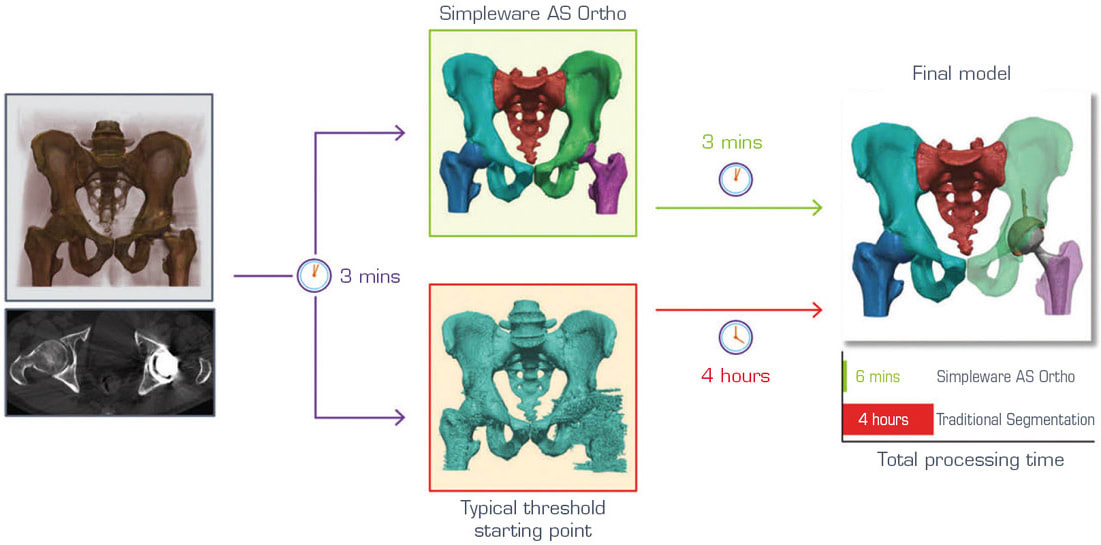
By comparison, planning revision surgeries means having to account for situations where an implant is already in the body, or where there is noise in the scan (see Figure 3). As a result, it is necessary to separate the metallic material of the implant from the rest of the patient’s anatomy, for example, to separate features such as the acetabular ball and implant stem. Again, manual approaches can be very time-consuming, taking multiple hours to achieve a result that is ready as a starting point for a clinician or medical device engineer. The automated solutions seen in products like Simpleware AS Ortho are able to take this segmentation time down to roughly three minutes. Although an automated method is unable to completely remove all manual work, it can cover the majority of basic steps to reach a point where only additional cleanup is needed to make sure that the model is accurate and ready for whatever the next stage of the project might be.
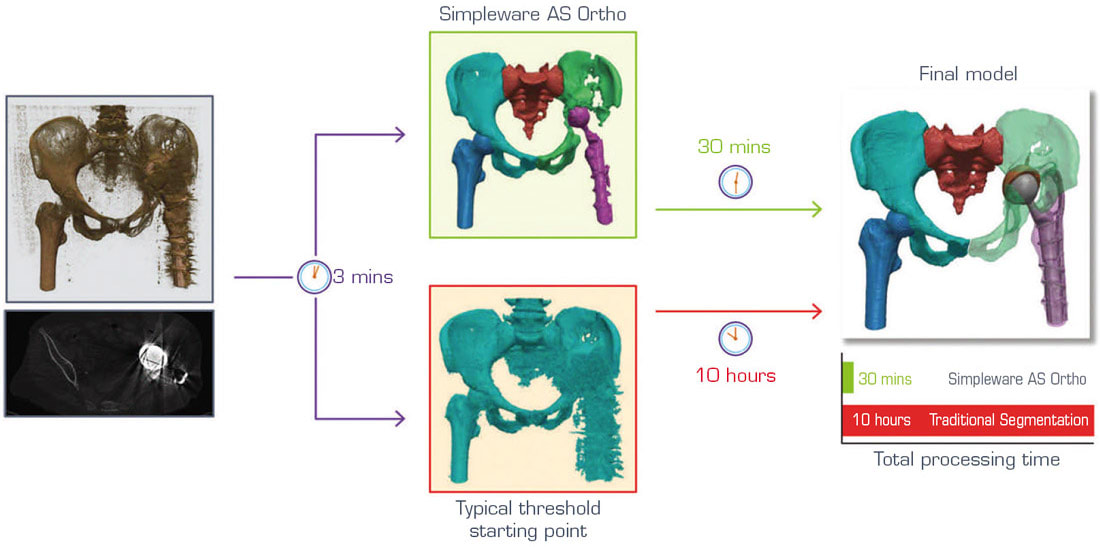
Another complicating factor in revision surgeries involves dealing with an unusual amount of noise in the scan data, or unique pathologies that cannot be handled by typical segmentation routines (see Figure 4). For these situations, automated segmentation is able to solve many manual tasks before a user has to work on the data. For example, this might include removing unwanted artefacts or scatter and is potentially able to reduce challenging cases from 8–10 hours to get to a workable model down to 30 minutes. It is therefore worth emphasizing how AI approaches do not completely automate revision and other surgical planning challenges but allows users to get to a place where much of the more tedious jobs have been dealt with.
Conclusions
The ultimate goal of using AI-based machine learning for orthopedic revision surgeries is to cut down on the headaches of manual segmentation and landmarking, even when working with more complex cases. Engineers, technicians, and clinical professionals working with image data can free up more time for higher-value tasks, including analysis and simulation of procedures. What this means for the patient is a shorter route from an initial patient scan through to a diagnosis or surgical plan. Getting the manual segmentation and editing down to a minimum also means that it is possible to rapidly generate models for computational simulation, something that is being gradually accepted by FDA for in silica trials, and to produce patient-specific 3D prints.
Of course, there are still future challenges to improving the accuracy and applicability of automated segmentation, including the availability of high-quality datasets and the scalability of solutions for different levels of healthcare. To this end, the technology behind software like Simpleware AS Ortho is now being applied to cardiovascular and other cases, as well as part of more comprehensive measurement and simulation workflows that benefit from automation.
This article was written by Dr. Jessica James, Business Process Analyst, Simpleware Product Group, Synopsys Northern Europe, Exeter, UK. For more information, visit here .
References
- Jiang F, Jiang Y, Zhi, H, et al., “Artificial intelligence in healthcare: past, present and future,” Stroke and Vasc Neuro, 2(4):230-243.
- Klug A, Gramlich Y, Rudert M, et al., “The projected volume of primary and revision total knee arthroplasty will place an immense burden on future health care systems over the next 30 years,” Knee Surg, Sports Traumat, Arthro, 1- 12, 2020.
- Haglin JM, Eltorai AEM, Gil JA, et al., “Patient-Specific Orthopaedic Implants,” Ortho Surg, 8(4):417-424, 2016.
- Turner T, “Hip Revision Surgery,” Drugwatch, 2020.