An exciting trend in drug delivery is underway: the movement toward smaller, smarter, wirelessly connected electronic devices that allow patient-administered therapy. Inspired by the technological advancements driving the consumer electronics market, new methods for drug delivery show great promise for all stakeholders. Patients wishing to claim more autonomy over their drug regimens, caregivers and medical professionals wanting to more closely monitor drug compliance, health insurance organizations looking to keep costs down, and developers of pharmaceutical products interested in conducting better-managed clinical trials can all benefit from these novel, next-generation technologies.
Smart Drug Delivery, Defined

Smart technology, as illustrated by the ubiquitous smartphone, refers generally to devices that feature complex but small electronics, high functionality, embedded software, wireless connectivity, and intuitive user interfaces that include graphics, LEDs, and buttons. When applied specifically to such drug-delivery devices as inhalers and medication-delivery pens, however, “smart” describes an evolution from purely mechanical operation to electromechanical operation. Self-administration of drugs by the patient and the availability of related smartphone and tablet apps for multi-device integration represent additional smart characteristics, as does the trademarked Bluetooth Smart technology, which refers specifically to the use of the Bluetooth® low-energy, power-efficient wireless protocol.
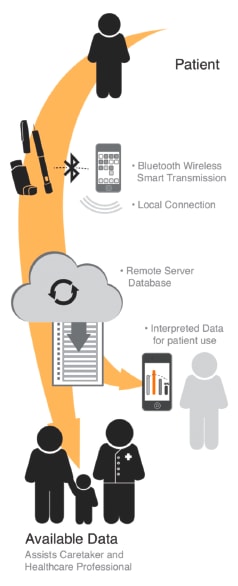
The numerous advantages of smart drug-delivery technology include improved therapies, reduced costs for both individual patients and the overall healthcare system, and increased patient compliance, which, in turn, should yield better outcomes. (See Figure 1)
Making Conventional Drug-Delivery Devices Smart
Popular, widely prescribed drug-delivery devices such as inhalers and insulin pens play critically important roles in the effective management of such chronic conditions as asthma and diabetes. These devices can be greatly improved for some—if not all—users, however, by the incorporation of electromechanics and smart technology.
A Better Inhaler: Electronics-based improvements to inhalers can put a new spin on—and add value to—more traditional, non-automated versions, such as GlaxoSmithKline’s Advair dry-powder inhaler. Electronically enhanced, more automated inhaler therapy can cater to and be optimized for patients who cannot breathe with sufficient force, such as infants and the elderly, or who have motor or cognitive disabilities. Historically, patients unable to effectively use inhalers have received treatment via nebulizers. However, this type of therapy is time-consuming, requires setup and cleaning after each use, and often requires an additional person to operate.
Smart enhancements to inhalers can include integrating a motor drive to advance and/or dispense the drug, adding a piezo electromechanical transducer for creating vibrations to aerosolize the drug, and designing in a breath sensor to automatically trigger drug delivery as inhalation occurs. Furthermore, a graphical user interface can improve ease of use and make fault messaging apparent; dose history can be electronically logged; and wireless transmission of dosage logs to a smartphone or Web viewer can be sent to the patient, caregiver, and physician. (See Figure 2)
With all of these changes to the existing inhaler, ease of use is vastly improved and patients representing a variety of ages, abilities, and environments can successfully operate the device. Family and healthcare staff can electronically monitor device use and dosage, intervening when necessary, and dependency on a nebulizer may be significantly reduced.

A Data-Centered Insulin Pen: Conventional mechanical insulin pens represent an improvement over traditional vial-and-syringe insulin delivery in terms of ease of drug administration, portability and convenience, and discretion. Like the standard metered-dose inhaler, however, the insulin pen could significantly benefit from a smart makeover through the addition of electronics and, especially, wireless connectivity.
For the diabetic individual, appropriate and timely insulin dosing are of paramount concern, and a smart insulin pen can help. Each dose of insulin administered from a smart pen is recorded and logged, with the last-dose timestamp and dose count shown clearly on the pen’s display. Dose history can then be wirelessly transmitted—typically via Bluetooth Smart technology—to a smartphone app and made available to the patient, caregiver, and medical professional. Additionally, programmable dosing, motor-driven dose delivery, and even a sleek docking station can enhance and facilitate the user experience. (See Figure 3)
Diabetes apps for smartphones and tablets have been around for a few years, and insulin pens incorporating electronics are currently on the market. However, the linkage of the pens to the apps provides the most benefit and is the newest innovation in this field. The right contract designer, developer, and manufacturer can assist pharmaceutical companies in bringing this type of connected, linked product to market.
Bringing Smart Drug Delivery to the Hospital
Electronically enabled, wirelessly connected, smart drug-delivery devices are well suited to home and on-the-go use. But their advantages are valuable within hospitals and other clinical settings as well. The intravenous (IV) pump, for example, is a clinical drug-delivery device that can benefit immensely from smart technology.
As more-sophisticated electronics are incorporated into IV pumps, the devices are better able to sense and measure how much fluid is falling. Built-in Wi-Fi technology ensures all measurements and data are sent to the nurse’s station and are even automatically recorded into a patient’s file. Drug and medical device companies looking to develop smart, connected, hospital-use drug-delivery devices such as these should ensure that they partner with a contractor well-versed in current regulatory requirements for this type of product, as issued by such agencies as the FCC and FDA.
Smart Partnering for Smart Products
Pharmaceutical companies seeking to explore this new frontier of smart, connected drug delivery are advised to partner with a contract design, development, and manufacturing company having a deep knowledge of and experience with such areas as cutting-edge smart technologies, embedded-software development, and ergonomic design.
More specifically, the partner company should offer proven experience and competence in the following areas:
- Wireless technology, particularly Bluetooth Smart
- Battery-powered systems and rechargeable systems
- Effective medical quality management systems
- Medical-grade embedded software development. This new generation of devices is powered by microprocessors, so there is software inside that’s running the device’s functions. Candidates for contract partnership must also be well-versed in related embedded-software development and validation regulation
- The systems-engineering approach to complex product development
- Mechanical design, plastic molding, and automated assembly
It is imperative for success that the partner company be focused on integrated engineering, development, and manufacturing of complex, patient-focused electro-mechanical drug-delivery devices. It also must be able to demonstrate that design and development, industrialization and new product introductions, scalable manufacturing, and end-use packaging are among its core competencies.
Summary
Adding electronics and connectivity to drug-delivery devices, particularly for drugs self-administered by patients outside the clinical setting, is a trend gaining momentum thanks to the associated wealth of advantages to patients, caregivers, medical professionals, and the greater healthcare system alike. Drug companies looking to bring these types of smart products to market as successfully and speedily as possible will benefit from partnering with a contractor that has the deep technical knowledge, talent, and well-honed processes required to do so.
This article was written by Bill Welch, Chief Technology Officer, and Chris Conger, Director Technology Development, Phillips-Medisize Corporation, Hudson, WI. For more information, Click Here .
MD&M Minneapolis, Booth 1027



