Ultrasound has long been associated with diagnostic imaging, but it is increasingly being explored and utilized as a beneficial, non-invasive tool for applications ranging from surgery to therapy. Researchers at the University of Michigan are focused on refining a new technique called histotripsy, which uses an ultrasound intensity hundreds of times higher than what is normally required for diagnostic imaging. “Histo” means soft tissue in Greek, and “tripsy” means breakdown.
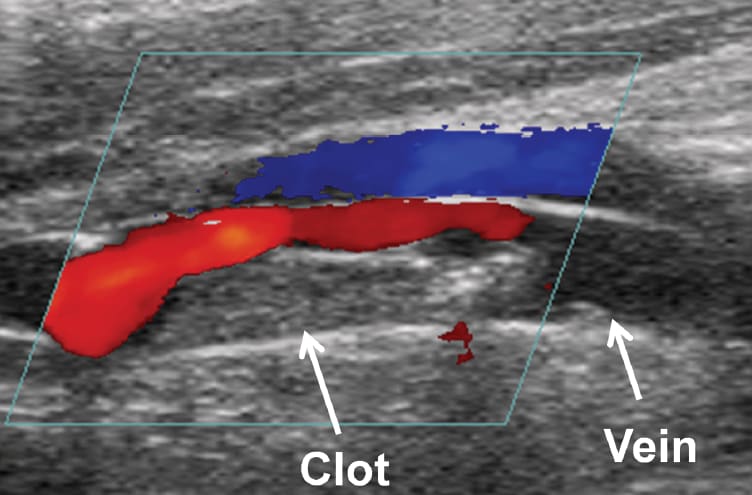
More recently, the histotripsy approach was extended for use in the breakdown of clots (thrombolysis) by the team of Adam Maxwell, Charles Cain, Hitinder Gurm, and Zhen Xu at the University of Michigan. In 2008, the team led by Dr. Xu was awarded a grant from the National Institute of Biomedical Imaging and Bioengineering (NIBIB), titled “Image Guided Non- Invasive Ultrasonic Thrombolysis Using Histotripsy.” This project is investigating the thrombolytic aspects of histotripsy for the non-invasive treatment of deep vein thrombosis (DVT).
DVT is a condition in which a blood clot forms in a vein deep in the body. It can lead to pulmonary embolism if the clot dislodges and travels to the lungs. It is more likely to occur in individuals over 60, but it can strike anyone. For some, an extended plane trip is enough to cause DVT. Sitting still for extended periods can cause blood to pool in the lower legs. For others, vein trauma from surgery, inflammation, or illness can cause blood clots. Only about half of the 2 million individuals who experience the condition each year have symptoms. Individuals may experience pain, swelling, tenderness, discoloration, and warmth at the affected site. Each year, up to 600,000 people are hospitalized, and approximately 300,000 Americans die from DVT-related pulmonary embolism in the United States.
To treat DVT, clinicians presently have two options. The first involves drug therapy to thin the blood and thus reduce the clot. The second choice is to invasively remove the clot using a plastic tube called a catheter. Both approaches carry a high risk of bleeding, and invasive procedures, such as catheter intervention, can also damage the blood vessel wall and cause infection.
Histotripsy may soon offer patients a third treatment option that is less invasive than current treatments while offering the added benefit of reduced recovery time. Current treatments for DVT require a 2- to 3-day hospital stay, but Dr. Hitinder Gurm, an interventional cardiologist and one of the collaborators with Zhen Xu and Adam Maxwell, states that histotripsy is 50 times faster than anything currently available. If this adaptation of the histotripsy technique passes all the necessary safety steps, Gurm anticipates that the procedure could be used as an outpatient treatment.
How Histotripsy Works
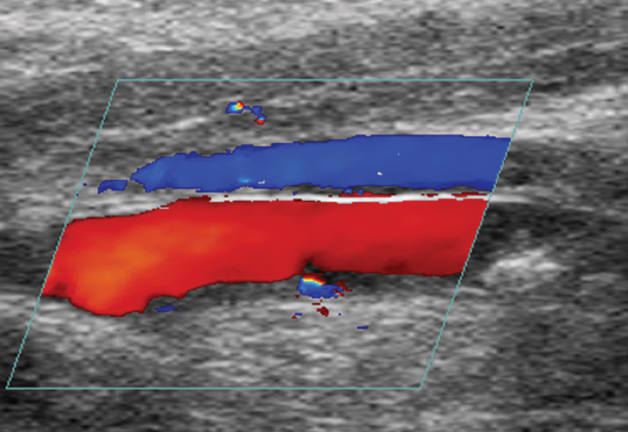
In the histotripsy system, an imaging transducer is closely aligned with the cloud-generating therapy transducer. This allows researchers to view microbubble cloud activity as it occurs. “We can see in real time when the cloud is generated, if it’s working, and if it has been effective in breaking up the clot,” says Xu. They also use color Doppler imaging to assess improvements in blood flow during the process.
A unique aspect of histotripsy is its influence on the cavitation process, which was previously considered uncontrollable. Xu and Maxwell achieve this precision using real-time cavitation monitoring and appropriate ultrasound pulse sequencing. The pulse sequence consists of a quick burst (less than 10 μs) of an ultrashort, high-pressure pulse. The pressures used to create cavitation are at least 10 times greater than pressures used in diagnostic ultrasound and comparable to pressure used in lithotripsy. “The idea is to generate the bubble cloud, fractionate a portion of the clot, and generate the seeds of the next cloud. All activity is finished before the next pulse arrives,” explains Xu.
A potential concern with clot removal is that fragments may travel beyond the clot site and create a life-threatening situation by blocking a key artery such as the pulmonary artery in the lung. In conventional treatment, physicians sometimes insert a mechanical filter into the vessel to trap stray clot material. But as Xu and her colleagues developed the histotripsy technique, they discovered a new phenomenon that may eliminate the need for filters. “The cavitating bubble cloud induces a fluid flow in the vessel similar to a vortex,” she says. By creating a second microbubble cloud a short distance from the clot, they may trap and completely dissolve any stray clot fragments.
Although this noninvasive emboli trap (NET) technique has only been tested in vitro, Xu anticipates it could have a number of clinical applications. “If we can make the NET work in vivo, it will open the door to new applications such as trapping clots during cardiovascular operations,” she says.
Xu and Maxwell’s early studies involved soft clots formed in plastic tubing and pig models, but they are now investigating histotripsy’s ability to fragment harder, mature clots. However, the structure of older clots is similar to that of the vessel, which presents new challenges.
“In these cases, it is difficult to differentiate between the clot and the vessel wall [because the clot has grown into the vessel wall],” says Xu. In this situation, the goal of the histotripsy is to create a channel through which the blood can flow rather than trying to fragment the entire clot. In preliminary animal studies, histotripsy did create a flow channel but required longer treatment times.
Next Steps and Future Clinical Applications
Future work will focus on four components. The first involves investigating the basic science behind microbubble-cell interaction; understanding the physical mechanisms responsible for these interactions will help optimize the technology. The second component will determine the safety and efficacy of the histotripsy technique. A third area will explore what mechanisms are at work with the NET phenomenon, including fluid flow patterns. The final area involves engineering and software development to prepare the system for clinical use. Xu notes that the goal is to automate the histotripsy technique so that physicians can sit down at a console; locate the clot with ultrasound imaging; lock onto a clot using a beginning, middle, and end point; press a button; and let the system scan the blocked blood vessel. Work also will focus on integrating the therapy and imaging transducers and reducing their size. The researchers will also examine ways to transmit the ultrasound energy without submerging the therapy transducer in water.
“We combine the best of two worlds: the noninvasiveness of drug therapy and the localization of catheter procedures, but without the complications associated with those two approaches,” says Xu. “If we are successful in creating a clinical system, DVT treatment could become an office-based procedure. In the long term, we can open the door to treating other conditions caused by blood clots as well.”
Since April 2010, Xu's team has made progress in two areas of clinical translation of the histotripsy thrombolysis technique. First, an initial series of animal studies were conducted in a pig deep vein thrombosis (DVT) model, successfully demonstrating the feasibility and safety of histotripsy. These experiments revealed that blood clots in the femoral vein can be removed by histotripsy without damaging the overlying tissue. No clinically significant adverse effects were observed. Second, a compact ultrasound therapy transducer integrated with an ultrasound imaging probe has been designed and built specifically for the DVT application. The therapy transducer has successfully passed their initial lab testing.
“Our work currently focuses on two main components aimed to accelerate the clinical translation and commercialization of this technology,” says Xu. “The first component is to build a complete, portable prototype system with integrated histotripsy thrombolysis therapy, imaging feedback for pre-treatment planning, treatment monitoring and post-treatment evaluation, and automated treatment control console. The second component is to determine the safety and efficacy of the prototype system and histotripsy-mediated thrombolysis in more extensive animal studies. We anticipate completing these components within a year.”
More Information
This work is supported in part by the National Institute of Biomedical Imaging and Bioengineering. For more information about this research, visit http://www.bme.umich.edu/labs/xulab or http://www.histotripsy.umich.edu .
References
Maxwell AD, Owens G, Gurm HS, Ives K, Myers DDJ, Xu Z. Noninvasive Treatment of Deep Venous Thrombosis Using Pulsed Ultrasound Cavitation Therapy (Histotripsy) in a Porcine Model. Journal of Vascular Intervention Radiology. 2011;22(3):369-377.
"DVT at-a-glance." PreventDVT.org: The Coalition to Prevent Deep Vein Thrombosis. Available at http://www.preventdvt.org/docs/pdf/DVTAtAGlance.PDF . Maxwell AD, Cain CA, Duryea AP, Yuan L, Gurm HS, Xu Z. Noninvasive thrombolysis using pulsed ultrasound cavitation therapy— histotripsy. Ultrasound Med Biol. 2009;35(12):1982-94.



