Custom integration of original equipment manufacturer (OEM) products can be complex, particularly for medical device integrators that build diagnostic instruments incorporating numerous optical components. Often, objective lenses, illumination sources, and imaging detectors are assembled and custom-mounted into finished instruments. Such components must not only meet stringent performance requirements, but often have to meet established Food and Drug Administration (FDA) standards.
Medical device design and manufacture includes traditional areas, such as the manufacture of stents, surgical implants, and other products used to diagnose and treat patients, along with instruments that aid scientists in areas such as genetic sequencing and cancer detection. Some devices use transmitted light microscopy and are able to employ advanced technology such as virtual microscopy or advanced cellular imaging techniques to provide images used by pathologists for diagnosis.
As performance, reliability, and other requirements evolve, the imaging field is changing and technology is advancing rapidly. In many cases, instruments have become highly controlled environments featuring self-contained, turnkey imaging systems that require minimal operator control. Automated optical components may be used to find focus quickly and repeatedly, making it possible for manufacturers to set up systems that are programmed to record image data for every sample.
Light Considerations: Performance, Objectives
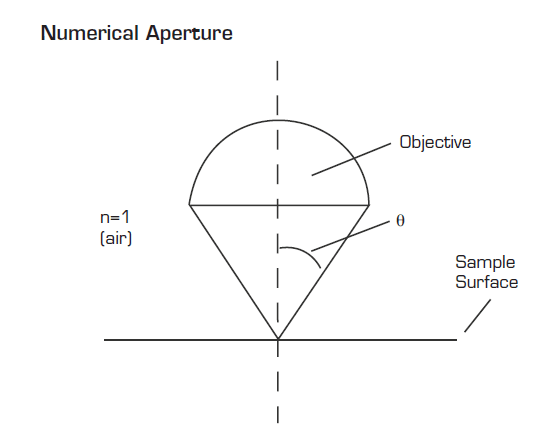
Performance specifications such as resolution, magnification, and transmission are vitally important for systems with optical components or microscopes. Lateral resolution refers to the smallest distance between two points on a specimen that can be distinguished as two separate entities — for example, distinguishing between two cells or intracellular features. Numerical aperture (NA), one of the most important specifications for any objective lens, refers to the light-gathering ability of the lens. Resolving power increases proportionally with NA and, generally speaking, higher-NA objectives carry a higher price tag as well.
Most objective lenses are designed for peak performance at a specific wavelength defined by transmission characteristics and chromatic aberration. Match ing system wavelengths to the appropriate objective is critical to overall performance. Many objective lenses are corrected for the visible light spectrum (400–700 nm). Other specialized objectives peak in the ultraviolet (UV700nm) ranges.
Fluorescence is one of the most useful techniques for imaging subcellular structures. Particular portions of a cell are tagged with a fluorescent label, which causes them to emit light of a specific longer wavelength when stimulated by photons of a particular shorter wavelength. When specifying systems for fluorescence and related imaging methodologies, additional components may be required, including filter cubes that specifically match the illumination source and objective. These cubes are paired with excitation and emission filters specifically designed to match the fluorescent tag.
One method of improving resolution in cell biology, anatomic pathology, cytopathology, and other fields is the use of immersion objective lenses. Resolution is dependent on numerical aperture defined by the equation NA = n (sin μ), where n is the refractive index of the medium between the objective and the specimen. The maximum NA using a dry objective is 0.95, where n=1. By using immersion media with a refractive index greater than 1, the NA of the optical system can be increased. As an example, typical immersion oil has a refractive index of 1.518, which will increase the NA to as high as 1.4. To use an immersion objective, the microscopist lowers the tip of the objective into water, specialized oil, silicone or glycerine, depending on the refractive index desired, and images the sample directly through the immersion medium. Immersion optics often requires the use of a coverslip and a specially designed correction collar that is used to adjust for the thickness of the sample.
Mechanical and Illumination Considerations
In specifying optics, mechanical and illumination considerations are also crucial. Systems may need to deliver a specified spatial resolution for optimal visual contrast when imaging or collecting spectral data. Most optical systems today use an infinity-corrected optical design, which allows intermediate modules and components to be introduced without affecting magnification. There is, however, a fixed range for the distance between the objective lens and tube lens.
Working distance (WD) is related to the utility and design of optical systems. It refers to the distance between the glass element of an objective’s mounting structure and a focused specimen. The longer the WD, the more space there is between the objective and the focused image plane. This provides two benefits — sample safety (the objective must travel further before it contacts the sample) and the ability to image a sample located within a defined package or holder such as a Petri dish or well plate. Typically, however, an increase in WD corresponds to a decrease in NA.
For those manufacturers integrating individual optical components, another consideration when selecting optics is the focal length of the optical system — the ratio of the focal length of the tube lens to the focal length of the objective lens. To change the total magnification of an optical system, it is often easiest to change the objective lens since there is typically more selection from manufacturers; in cases where there is a specific objective lens performance characteristic that must be preserved, the tube lens may be changed.
Depending on the system, the illumination source may be a laser, LED, or standard halogen or mercury bulb. But even the selection of an illumination source sometimes can affect the components specified. Both the mechanical interface of the entire optical system and its total demands are important factors, even when they do not appear to directly affect performance. Every optical component and detector is rated to withstand a certain amount of energy; integrators working with high-power energy sources such as lasers should check with the manufacturer of all components for compatibility. In addition, many samples are temperature-sensitive. As a result, the experimental apparatus often is designed to remove heat generated by the power or light source. In many cases, using an external cold-light source with a fiber-optic connection is an optimal solution.
Imaging Considerations
Cameras and other imaging detectors are another key component of today’s medical device diagnostic systems whenever viewing, capturing, or archiving images is required. In addition, integrators of the future may have to ensure that images they collect will be suitable for uploading to laboratory information management systems. Some questions to consider when selecting a suitable digital imaging device include:
How cool does it need to be?
Depending on the application, some cameras require cooling. Cooled cameras are more sensitive at low light levels and therefore are particularly well suited to fluorescence imaging, but are usually also more expensive. Liquid nitrogen, water, air, or Peltier thermo-electric systems can be used to cool camera components. Some of these choices require additional space or external equipment.
Will it deliver the images I need?
Some people mistakenly believe that more megapixels mean better-quality images. The truth is that designers must match the optical resolution of the microscope system or spot size projected on the charge-coupled device (CCD) detector with the digital resolution of the camera to avoid over- or under-sampling. The spot size of the projected image depends on the objective lens magnification, numerical aperture, tube lens, and camera mount magnification. The resolution of the CCD varies based on the size and number of pixels. The desired optical resolution should be considered first, and then digital spatial resolution should be determined, as it is the limiting factor in achieving the system’s overall resolution. This phenomenon is explained by Nyquist’s criterion, which says that twoto- three pixels on a CCD chip are required to resolve the smallest feature the optical system is capable of reproducing. Using a smaller number of pixels results in image degradation. A larger number of pixels does not assist in resolution and may make it more complex and costly to share, transmit, and store images.
What image analysis or other software capabilities do I need?
Digital cameras can be used either with off-the-shelf or integrator- designed software. Integrators typically look at ease of use, efficiency, the ability to document system parameters, and the delivery of reliable results in considering whether and how to design software. With the growth of automation, hardware components are increasingly becoming motorized. In - tegrators need to take into account the compatibility of all components in tandem, in order to develop code that can optimize the combined capabilities of all system elements; in addition, the software must be customized to suit the needs of life scientists and medical professionals.
Conclusion
With knowledge and careful planning, system integrators can select optics, filters, light sources, and cameras for their medical diagnostic instrumentation. With the requirements of the FDA becoming more stringent, integrators need the support of a trusted optical supplier that adheres to a comprehensive quality program for the manufacture of its high-performance optical components. But while suppliers have a plethora of data about performance, not all of that data is in the public domain. Select a supplier that deeply understands the business requirements, work together closely over time, and be prepared to provide detailed requirement parameters, which will allow the supplier to specify whether a given optical component will meet your needs. The supplier also needs to understand logistical needs and should be able to handle delivery requirements once the product is on the market. This will ensure the delivery of products that meet both the manufacturer’s requirements and those of its customers.
This technology was done by Jennifer Wrigley, Olympus America Inc, Center Valley, PA. For more information, Click Here .



