The smart factory has been a top manufacturing initiative for years as shop floors continue to become digital, automated, and intelligent. Today, companies are investing heavily in smart manufacturing, intelligent automation, and advanced robotics. A 2019 report from PricewaterhouseCoopers and the Manufacturing Institute found that 73 percent of manufacturers planned to increase their investment in smart factory technology over the next year.1
Even as Industry 4.0 and its underlying technologies increase in various areas of manufacturing, many manufacturers still struggle to digitize the “last mile” of their factory floor, where operators typically still use paper-based device history records (DHRs), or production records. No matter what other investments in plant digitization have been made — enterprise resource planning (ERP), manufacturing execution systems (MES), material requirements planning (MRP), learning management systems (LMS), the list goes on — the investments are too often impeded by critical processes such as DHR execution and review that remain manual, disconnected, and often paper-based activities.
Paper may seem like a cost-effective option for managing DHRs, but even a small amount of it on the factory floor carries high costs associated with quality alone. For manufacturers, risks of relying on paper-based processes in a data-driven world include disconnected and inefficient DHR processes, inaccurate information on the DHRs, poor data tracking throughout the production life cycle, preventable quality issues and costly delays. In a survey of senior executives from medical device, contract manufacturing, and pharmaceutical companies, Accenture found that quality testing and batch release accounts for upwards of 70 percent of manufacturing lead time mainly due to manual processes, disconnected instruments, and nonstandard paper-based documentation and control procedures.2
Simply put, paper on the last mile of production — FDA 21 CFR Part 820 review and product release — remains a barrier even for manufacturers making strides in their digital transformation efforts. When people on the production line are left to rely on paper, line workers and quality teams are forced to rely on inefficient processes, outdated tools, and inadequate data. With just one missing link in the technology chain, that smart factory future can feel far removed from day-to-day operations.
Digital technology can help manufacturers bridge the paper gap between quality and manufacturing to improve operational efficiencies and product quality during the last mile of production (see Figure 1).
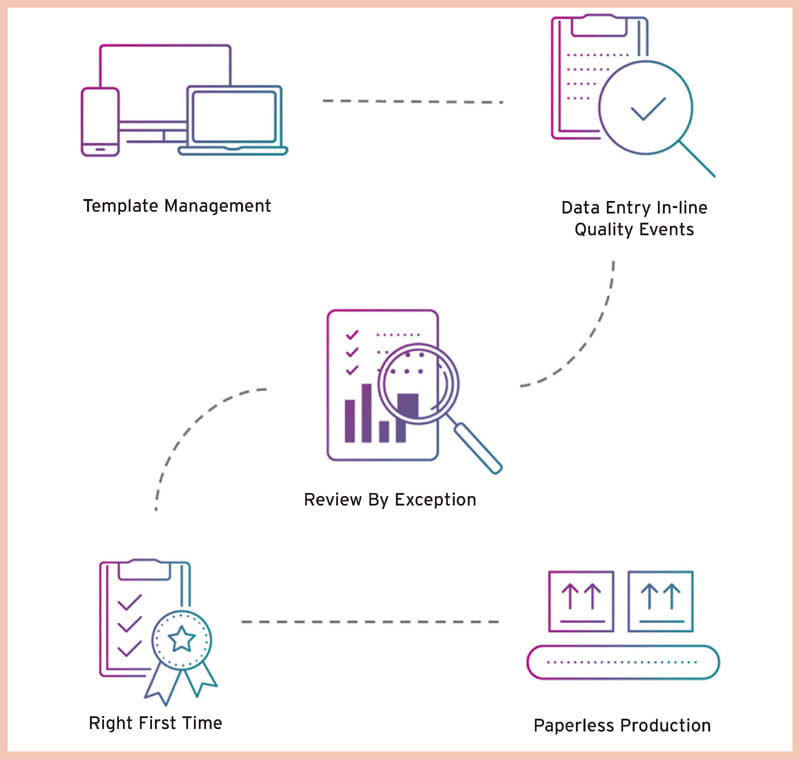
How a Digital DHR System Can Bridge the Production/Quality Gap
While many medical device manufacturers have already implemented Industry 4.0 or digital transformation initiatives, they continue to rely on a variety of paper-based or disconnected information systems to monitor, collect and aggregate data surrounding production and quality processes. Poor synchronization between these disconnected data sources severely limits the throughput between manufacturing, quality and other critical business areas, which tends to impede physical operations. As a result, these manufacturers struggle to connect their machines, enterprise systems and the data produced by these entities to access actionable performance insights.
To capture the data and insights needed to optimize production, reduce deviations and corrective/preventive actions (CAPAs), improve right-first-time (RFT) metrics, and accelerate product release, manufacturers must integrate their DHR processes with their ERP, MES, LMS, and other manufacturing systems for a more complete view of the data within their business.
With a paperless DHR system, medical device organizations can create productive connections between systems, processes, and people throughout the entire manufacturing organization for a holistic view of data. Machine operators can input data directly into tablets or computers on the shop floor and pull information directly from an ERP system. They can link standard operating procedures (SOPs) and work instructions to DHR phases, launch deviations, CAPAs, and other quality processes directly from the DHR for constant visibility, automatically launch and enforce training on the production line, and ensure equipment compliance in real time.
By integrating critical manufacturing applications such as ERP, MES, MRP, and others with a digital DHR solution, manufacturers can leverage their data in ways that a paper-based, disconnected system won’t allow:
Collect data. Easily configure the type, timing and frequency of data to be collected, eliminate errors in data input and transfer, and ensure data integrity as data moves between systems.
Connect data sources. Integrate the DHR process with other systems, seamlessly share data between those systems in real-time, and consolidate bill of materials data in a single system.
Contextualize data. Guide operators and enforce limits, thresholds, and training with integrated, data-driven prompts, and gain real-time visibility into production lines, batches or lots.

Collecting, connecting, and contextualizing data needed by both quality and manufacturing teams helps address shared processes like document revisions, GMPs, training, testing/sampling, and review and release. By fully integrating production and quality digitally, manufacturers gain the actionable performance insights necessary to improve processes and mitigate errors of manual, paper-based DHRs.
How a Truly Paperless Last Mile Can Improve Operations
Considering the dynamic nature of a factory floor and the number of people who need to collaborate and share information, replacing paper-based records with digital records has the potential to fundamentally transform operations in three key ways.
Easily Manage Templates. By digitizing their production record processes, manufacturers can configure the DHR system to their needs. A digital system allows them to easily build and accurately maintain digital master record templates, which virtually mimic paper-based records, with tools to capture many different data types, perform calculations and time capture, enable configurable limits and controls, and easily include attachments. For example, if a manufacturer’s processes need to be performed for a certain duration (e.g., at least 1.5 hours), digital tools can automatically record “time in” and “time out” to confirm the processes were performed for the required amount of time.
The digital output will be visible in real time with the familiar look of paper records. Templates and global elements can fully automate change management. Altogether, a paperless DHR process can reduce time spent building templates from months to days. According to one life science company’s initial forecast, digital production records will result in 30 percent employee efficiency gains while scaling manufacturing.
Enter Data Completely and Correctly. The data-input errors common with manual, paper-based DHRs — incorrect, illegible, or missing entries — can have a ripple effect as data advances through the production process and create data integrity issues across the organization. Bad data on the DHR leads to deviations and delays so the organization must focus on quality control efforts and expenditures rather than value-added activities.
A digital DHR system lets users securely collaborate on DHR processes, with parallel and sequential processing and FDA-compliant e-signatures. Line workers can easily capture all entries in real time so the data is immediately available for reporting, and automatic data-integrity checks will ensure the data is entered completely and correctly. This means manufacturers can error-proof data input, digitally authenticate users, and automate review/traceability of changes on the record.
According to Corona, CA.-based contract manufacturer Wellington Foods, digital production records have facilitated a more than 90 percent reduction in data input errors and data integrity issues on the shop floor. Better data has driven fewer deviations and shorter documentation review cycles.
Review DHRs By Exception. Where a digital solution can help manufacturers most is in quality review of the DHR, where it can significantly reduce traditionally long review cycles and lead time from batch/lot completion to product release. With a paperless solution, there is no manual, page-by-page searching for information. Rather than review the entire record for deviations, a manufacturer only has to review a subsection. There is concurrent quality review and production, as well as the ability to review and release remotely, to enforce quality controls without slowing production. Data-entry errors are corrected in real-time during production rather than after, e-signatures are always captured, dates and times are always correct, and training is always current.
According to EpiBone, a Brooklyn-based regenerative medicine company, a digital DHR solution’s review-by-exception functionality allows the company to close out a production record in 30 minutes, compared with the two weeks it previously took to sign off on paper records. Whereas making revisions could take a month or longer on a paper-based system, now it takes about a week.
Digital DHRs: Paperless, Error-Proof, and Connected
As Industry 4.0 drives manufacturers toward digitization and data exchange in manufacturing processes, digital transformation on the factory floor is already reshaping how production processes and records are managed. By connecting users across the shop floor and eliminating manual data management, leveraging a digital DHR solution has shown to increase shop floor productivity so manufacturers can focus on their product rather than on their paperwork.
On average, life sciences manufacturers that have gone fully paperless on the shop floor with a digital production record system have seen a 90 percent decrease in data input errors, a 21 percent reduction in total deviations, and a 75 percent reduction in post-production review.
Of course, digital transformation isn’t as simple as buying and implementing new software, and a digital DHR system isn’t a silver bullet. An automated DHR solution extends rather than replaces existing data systems, such as MES, MRP, or ERP. What a digital DHR solution does for manufacturers that these systems can’t is to remove the remaining paper from the last mile of production, prevent bad data from being entered at the source, and enable productive connections between manufacturers’ systems and people. The cumulative effect is that manufacturers can ship products faster with full confidence in quality.
References
- “Navigating the Fourth Industrial Revolution to the Bottom Line,” PWC, 2019.
- “‘Dare to be Different,’ It’s time to revamp collaboration in life sciences contract manufacturing,” Accenture Life Sciences.
This article was written by Matt M. Lowe, Executive Vice President of Product, MasterControl, Salt Lake City, UT. For more information, visit here .




