Biosensors and point-of-care devices are poised to change the delivery of healthcare. Biosensor technology can be used in cheap, disposable point-of-care devices, or it can be used to provide continuous monitoring in implantable devices. Measurement in biomedicine presents unique challenges in both implementation and interpretation.
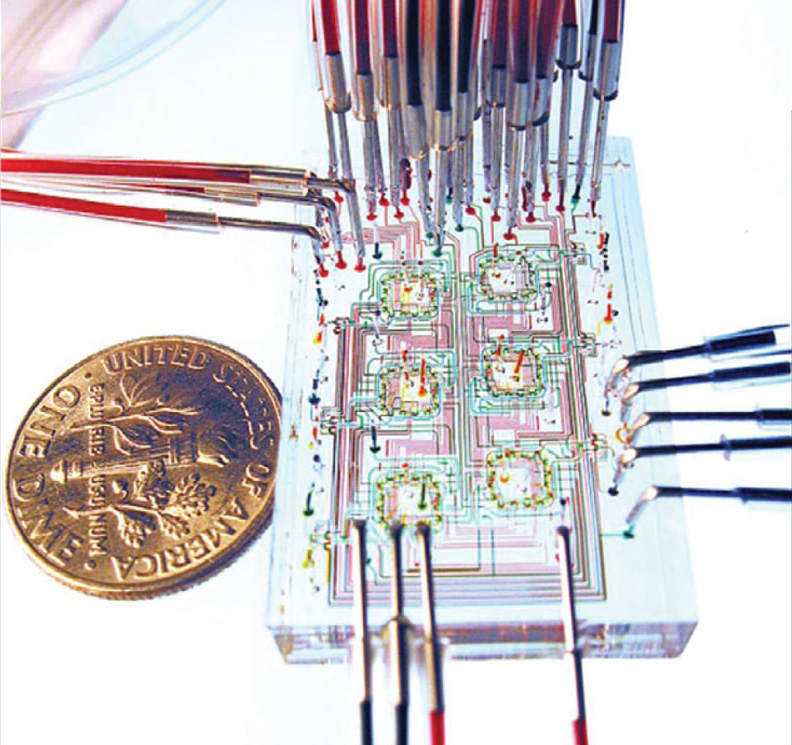
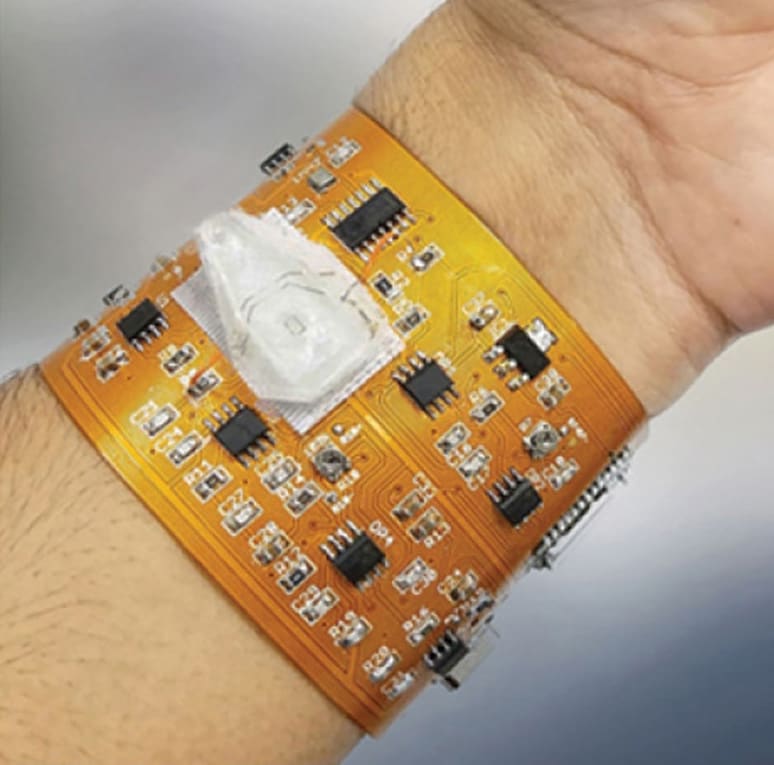
When biosensors are integrated into wearable devices, bio-markers can be monitored noninvasively in samples such as saliva and exhaled breath condensate as well as minimally invasively in blood and interstitial fluid using smart wristbands. This article explores cutting-edge developments in biosensors and examines their role in the future of healthcare. It also presents examples from current research.
What Are Biosensors?
A biosensor converts a biological reaction into a readable signal by biological materials. Using biological components, such as DNA or proteins (e.g., antibodies or enzymes), it detects a target analyst — the biomarker. The biomarker can be biological material like protein or DNA, or it can be nonbiological such as glucose. The reaction is typically converted into a readable signal by one of two methods: an electrochemical transducer or an optical transducer.
“Lateral flow assays and electrochemical test strips are the more traditional methods that currently dominate the market,” says Luyun Jiang, PhD, technology analyst for market research firm IDTechEx. “Lab-on-a-chip and molecular diagnostics are the emerging new technologies. Lateral flow assays use a capillary reaction to move the samples and to detect the binding of antibodies or antigens. This method is very fast, cheap, and simple and is widely used for pregnancy tests or infectious disease testing,” she says.
Electrochemical test strips are used for applications such as glucose testing for diabetic management. Using the blood from the finger to measure the glucose level has been around for a long time, and researchers are looking at wearables or implantables that use biosensors for continuous glucose monitoring, Jiang says.
Lab-on-a chip devices are based on microfluidic technology that often integrate different tests into a single device. These miniature devices automate the test process and enable true point-of-care biosensors (see Table 1).

Applications
Biosensors are ideal for monitoring devices that are used frequently such as diabetic glucose monitoring or for diagnostics such as pregnancy and fertility tests. They are also suitable for lifestyle devices such as those used to monitor cholesterol levels, for example, Jiang says. Biosensors are also integrated into diagnostic devices for cancer and genetic testing.
“Biosensors are already moving healthcare from being centralized in a lab to being decentralized at the point of care,” says Jiang. “Traditional biomedical diagnosis collects a sample from the patient (e.g., blood, urine, hair, or genetic sample) and sends it to an off-site analytics lab. The patient must return after a few days for their results. With point-of-care testing, the patient gets results within a couple of hours.” But this method is not only about saving time, she says. “Sometimes it means saving a life. A fast detection of the pathogen allows time to respond and then control the spread of the disease and avoid epidemics.”
Molecular Diagnostics
Molecular diagnostics are used to asses an individual's health condition at the molecular level, analyzing specific genetic sequences in DNA, ribonucleic acid (RNA), or the material or protein that is encoded in these genetic sequences, says Jiang. This testing follows the same principle as other biosensors, with a biomarker to be detected by a bioreceptor, which is transduced into a readable signal. Currently, this testing takes place outside of the body, therefore it is classed as a subset of in vitro diagnostics.
“Biomedical diagnostics and biosensors are becoming more important due to the aging and growing global population as well as the increasing concerns brought on by chronic lifestyle diseases,” says Jiang. “Emerging molecular diagnostics will have a big impact on our healthcare system.”
Biomarker-Based Screening
“One of the most promising approaches for making early disease diagnosis possible is to enable the idea of biomarker-based screening,” says Mehdi Javanmard, PhD, assistant professor, Department of Electrical and Computer Engineering at Rutgers University. “A biomarker is any type of molecular indicator that signals the presence of a disease or indicates whether a patient has a predisposition to getting that type of disease in the future.” Other promising biomarkers are protein biomarkers (e.g., PSA blood tests, pregnancy kits) and metabolites, which provide valuable information about what is happening at the metabolic level, he says.
One of the key advantages of biomarker-based screening, he says, is that in addition to enabling early disease diagnosis, they enable precision and personalized medicine — the ability to provide a diagnosis that has a much higher probability of being successful based on the patient's molecular information rather than diagnosis based on outward physical symptoms. Miniaturized technologies for detecting and monitoring biomarkers also opens up the potential for continuous health monitoring.
Javanmard's lab at Rutgers is developing miniaturized portable and wearable sensors that can monitor various biomarkers for continuously monitoring human health in real time. They are investigating molecular markers with measurements of various parameters such as temperature, heart rate, electrolyte concentration, dehydration, and glucose levels that already can be easily collected.
“This data can be integrated with data from pedometers, ECGs, or accelerometers for detecting things like hyperactivity. One of the most promising technologies moving in this direction are lab-on-a-chip technologies — or microfluidics — the ability to miniaturize instrumentation and techniques that are typically performed on a large scale in a lab. In addition to miniaturizing the chip, you also need to miniaturize the instrumentation that moves the fluids,” says Javanmard. “Part of that involves developing new fundamental electrofluidic components such as small electronic biosensors, as well as working on electronic and electromagnetic mechanisms for the pumping of fluids and applying those toward particular application and domain specific systems or platforms.”
Javanmard's lab is investigating biosensors for both human health monitoring and for studying the effects of environment on physiology. For human health monitoring, the lab is developing a fully integrated portable system for detecting protein biomarkers.
“It's not just miniaturizing the channels, but it's also a matter of miniaturizing pumps, so we're doing on-chip microfluidic pumping and on-chip electronic detection of the proteins,” he says. To do this, he says that multiplexing — the ability to analyze multiple biomarkers simultaneously — is critical. “We've come up with an electronic method for barcoding microparticles. The idea is to inject a test sample into the microchannel. The capture region has a cocktail of antibodies targeting different antigens. We capture these different antigens or protein biomarkers and then load barcoded beads. There's a one-to-one correspondence between the barcode and the protein biomarker. Each barcoded bead has a secondary antibody on it that targets a particular antigen. We elute the specifically bound beads. Downstream we have an electrical sensor that quantifies the beads and recognizes the barcode on the bead. As a result, you're able to detect quantity of protein and type of protein,” he says.
The advantage of doing this approach electronically, he says, is that miniaturizing the instrumentation becomes much simpler and more straightforward compared with using optics or fluorescence. The team was able to miniaturize the locking amplification circuitry required for impedance cytometry all onto a device the size of a wristband. He explains that as beads are passing through the electrodes, they are measuring the peaks. “We integrated the wireless transmission capability and developed a smartphone app for capturing data. We also developed electronic technology for capacitively barcoding the particles. That way we can still do everything electronically whereas other barcode techniques require optics for readout. Another project his lab is developing monitors the effects of environment on physiology. “Asthma is inflammation of the airway that results from environmental triggers. An important method for diagnosing this would be to measure bio-markers in exhaled breath (the droplets contained in breath),” says Javanmard. “We're interested in detecting nitrite content, which is a marker of inflammation in the respiratory airways.”
Their system detects nitrite in exhaled breath content using reduced graphene oxide, an exotic material that is bio friendly but also very sensitive. He says they were able to quantify the amount of nitrite in the exhaled breath contents.
Micro RNAs and Microneedle Arrays
“Analytical chemistry and biological systems present formidable challenges to very highly compartmentalized non-homogeneous systems very localized production down to the level of the cell or below subcellular components or even single molecules,” says Danny O‘Hare, PhD, reader, sensor research in the Department of Bioengineering at the Imperial College of London. “As the technology improves, lower and lower concentrations seem to be biologically important. We're trying to do all of this against a complex moving matrix.”
O’Hare says that electrochemical biosensors are attractive because they are cheap, and cheap is important because they get measurements at scale. MicroRNAs, he says, represent an interesting new class of biomarker that are challenging because they are 10-20 bases long. “By using PNA (peptide nucleic acid) instead of DNA, we get lower errors in hybridization. We use the hybridization to bring together two short PNA probes where they can then react, and the resulting chemical reaction fluoresces.” O’Hare's team is implementing this approach for a microRNA that is specific for prostate cancer.
His team is also investigating solid microneedle arrays for monitoring antibiotics. The arrays are part of a closed loop system for controlling drug delivery to optimize antimicrobials to minimize the risk of resistance. In the device, B-lactamase catalyzed hydrolysis causes a drop in pH. The next step, he says, is to extend the range of analytes and increase the signal as the binding of the target brings the electrochemical closer to the surface.
Conclusion
From lateral flow assays to lab-on-a-chip microfluidics, the advent of biosensors is moving diagnostics from the lab to the point of care. Doing so presents unique challenges in both implementation and interpretation, but cutting-edge developments are overcoming the hurdles and changing the delivery of test results.
This article was written by Sherrie Trigg, editor and director of medical content for MDB. It is a synopsis of a webinar presented earlier this year titled, Biosensor Technology: Driving Advances in Healthcare.” To view the webinar, go here .



