According to the American Chronic Pain Association, more than 50 million Americans suffer from some form of chronic pain. The most common types of pain include arthritis, lower back pain, bone/joint pain, muscle pain, and fibromyalgia. Therapeutic ultrasound is a noninvasive, drug-free treatment used for musculoskeletal and joint pain.

At the end of February, the FDA granted 510(k) clearance for SAM®, from ZetrOz, Inc., Trumbull, CT, the first long-duration, wearable ultrasonic diathermy device for applying deep therapeutic treatment.
The device uses high-frequency ultrasonic excitations to produce deep mechanical stimulation within the body, increasing circulation, reducing inflammation, and pushing nutrients through the body’s cellular structures, explains product inventor and company cofounder, George K. Lewis, Jr., PhD, who also serves as the Chair of Therapeutic Ultrasound for the American Institute of Ultrasound in Medicine. Here he explains the backstory, and current state of wearable ultrasound.
An Electrifying Beginning
I was raised on ultrasound technology. My father, Dr. George K. Lewis, Sr., a distinguished scientist and engineer in biomedical ultrasonics and array design, had long experimented with ultrasound technology and holds copatents with me on variations of the miniaturized device technology. Before I was in high school, I was working in my father’s R&D facility assisting him on acoustic measurements, transducer soldering, and becoming integrated into the ultrasound community.
Once I took interest in the therapeutic properties of ultrasound in graduate school, he helped me pursue my goal of miniaturizing ultrasound for self-administered pain relief. While pursuing a doctorate degree in Biomedical Engineering at Cornell University, I started integrating ultralow impedance ultrasound miniaturization technology into my academic and industry research and began exploring commercialization efforts to include the technology in noninvasive surgeries, pre-natal imaging, cancer ablation, and wound healing.
I attended Cornell as a presidential life science fellow, with a full scholarship to pursue a PhD in the biomedical engineering. In my first year, I became interested in brain cancer and other neurological diseases, and formed a thesis committee around developing a novel ultrasound-based drug delivery strategy that employed convective-drug infusions directly into the brain, coupled with ultrasound-controlled distribution of the neuronal therapy.
My PhD committee consisted of biomolecular and chemical engineering, neurobiology, and mechanical engineering professors that were supportive of my idea—however, I had to develop the equipment and experimental techniques to design, test, and evaluate my proposed ultrasound-based drug delivery device. I was fortunate to have also received research support from the National Science Foundation and National Institutes of Health, which allowed me to begin to design the first “ultrasonic drug delivery needle” and the associated driver and control electronics of the system.
While I was in the process of building the system and following engineering design notes available in the literature, I was accidently electrocuted with more than 1,000 volts from a high-power ultrasound driver circuit that I designed. After getting thrown back to the floor, feeling dizzy and nauseous, and being run through a whole suite of cardiac tests by the EMTs—I had my epiphany. To design a low-voltage, ultralow-impedance ultrasound system that is safe yet powerful…and with that, the technology was invented.
The first commercial product, a wearable ultrasound device for pain, was prototyped in 2008 and launched for commercial distribution into the medical market in 2014 with the help of my father. The battery-operated ultrasound device was later refined into various configurations for a number of biomedical applications— this included the wearable therapeutic ultrasound device.
ZetrOZ was awarded a few prestigious grants to bring this technology to fruition. These include a recent NIH Grant for $397,000, a Connecticut Innovations investment of $1.3 million, $100,000 award for primary healthcare innovation, and $50,000 as a runner-up in the 2011 Creative Core Emerging Business Competition. Along the way, we have also received a number of awards for the technology, including Most Impactful Technology awards, the Empact 100 Award and Most Innovative Research awards.
How It Works
Ultrasound is a mechanical wave that enhances the body’s natural process of providing circulation to tendons, ligaments, muscles, etc. Biological tissues are poroelastic and are similar to a sponge. The transport of fluids is increased when mechanically stimulated with compression and rarefaction forces. Ultrasound, non-invasively, acts on tissues in these ways by squeezing and opening up tissue, moving nutrient molecules to improve the cellular environment, and increasing total transport kinetics in tissue via mild, painless warmth. Its effects are subtle, taking time via daily “slow release” which makes the technology so effective.
Going deeper into the technology, ultrasound works via the mechano-transduction of cellular pathways, elastically perturbing the tissue and changing the permeability of tissues, nerves, cartilage, ligaments, tendons and muscle. The patient feels a soothing, thermal and numbing sensation as a flood of nutrients is transported in and the “waste” is removed. Vasodilatation due to local nitric oxide release and thermal regulatory effects of tissue broadens the utility of ultrasound. The therapeutic modality can also create a biological cascade causing angiogenesis—the stimulation of new blood vessel growth.
Ultrasound has been used safely and effectively for years to provide pain relief and reduce joint contracture (stiffness). Additionally, the thermal mechanisms triggered by ultrasound have been shown to facilitate wound and bone fracture healing, promote the penetration of topical ointments into the skin, and increase local circulation with a cascade of therapeutic biological benefit. Despite the potential of ultrasound therapy for treatment of pain and for healing, the therapy’s mode of delivery, ease of safe and effective use, size of device, and price have traditionally prohibited its broad translation to the public.
The new ZetrOZ ultrasound delivery system relies on aggressive miniaturization and integration of the ultrasound transducer, electronics, and power supply to produce a smaller, low-intensity ultrasound system that can deliver portable, convenient, and effective therapy for long durations.
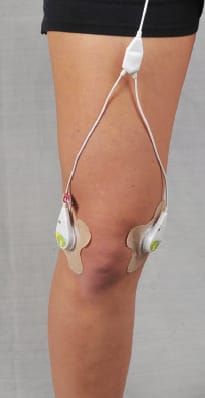
The device, called SAM, is the size of an iPod® and just as easy to use. It is coupled to the body with the first ultrasound bandage, like an oversized Band-Aid®, integrated carrier and ultrasonic transmission media. (See Figure 1) The coupling bandage contains a pre-measured, sealed cap of gel ultrasound conductor. The bandage ensures that the gel is in direct contact with the affected area and that the device is positioned appropriately on the body.
The patient or provider simply connects the applicator to the power controller jack, presses the applicator into the bandage and turns the device on to begin ultrasound therapy. (See Figure 2) A patient can receive treatment for approximately four hours, four to seven times a week. The current standard of care for ultrasound pain treatment is just one treatment per week, usually for approximately 15 minutes.
Where It Stands
The wearable long-duration ultrasound device for humans is continuing in clinical trials. We are in the midst of several pain and healing studies in conjunction with institutions such as Brigham Young University, Ohio State University, Duke University, University of Connecticut, and SUNY Upstate Medical Center. The milestones of the various clinical initiatives include refinement of the device for the treatment of knee osteoarthritis, upper back pain and healing soft tissue due to overuse injuries, and general clinical evaluation of daily treatment to improve patient quality of life and reduced pharmacotherapy use.
Preliminary studies conducted by the investigative team suggest that daily wearable ultrasound treatment provides an effective non-pharmaceutical approach to arthritis and back pain management, as well as healing tissues and reducing inflammation.
Thus far, the response from human clinical trials has been very positive in reducing pain severity, duration, and prevention. In a clinical trial on patients with chronic pain of the upper back, the device had a significant reduction of pain compared with placebo-control devices. Additionally, the subjects involved in the study were able to successfully self-treat, every day during normal daily activity.
In another clinical trial recently concluded in December 2013, the device demonstrated sustained deep therapeutic benefit in muscle tissue for multiple hours. Compared with placebo control, the active device achieved a quadrupled and doubled bioregulatory up-regulation of diathermy at 1.5 cm and 3.0 cm tissue depths, respectively for multiple hours, which is unprecedented in the therapeutic industry.
The company received FDA clearance for commercial distribution in November 2013. The wearable ultrasound device is in a new class of medical ultrasound therapy systems for wearable long-duration treatment, which is a momentous milestone. Before this device, ultrasound therapy was only cleared by the FDA for a maximum of 30 minutes of treatment.
Now that the device has received FDA 510(k) clearance, we expect that, by early summer, the device will be widely available in the US by prescription and over the counter in Canada. We anticipate a very early adoption by sports rehab and physical therapy centers, and a following of chronic pain management clinics using the device to help treat their patients. In the US alone, well over 200 million people could effectively use wearable ultrasound to help manage a painful situation without the use of pharmacotherapies.
Injury prevention or “pre-hab” is ideal for elite athletes and older athletes who have a need to protect their joints and rehabilitate on a regular basis. We also see trainers and other physical therapy professionals using wearable ultrasound technology to increase circulation, strengthen tissue, and stimulate some of the body’s other natural processes to help prevent injury to tendons, ligaments and muscle.
This article was written by George K. Lewis Jr., PhD, Chief Scientific Officer, Inventor, and Cofounder, ZetrOZ, Inc., Trumbull, CT. For more information, visit http://hotims.com/49745-167 .



