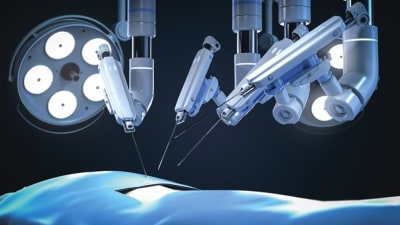
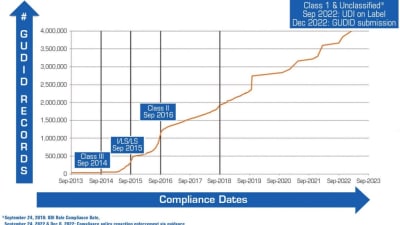
Stories
Briefs: Medical
Products: Medical
Products: Medical
Briefs: Medical
Briefs: IoMT
Briefs: IoMT
Supplements: Materials
Supplements: Regulations/Standards
Briefs: Connectivity
Features: Medical
Supplements: Sensors/Data Acquisition
Supplements: IoMT
Features: AR/AI
Supplements: Mechanical & Fluid Systems
Products: Electronics & Computers
Features: Regulations/Standards
Features: Wearables
Features: RF & Microwave Electronics
Features: IoMT
INSIDER: Medical
INSIDER: Wearables
Features: Test & Measurement
News: IoMT
FDA to Address New “Quik” Review Program Pilot for Faster Review of Certain 510(k)s
Features: IoMT
Briefs: Wearables
Features: IoMT
Features: Medical
Features: IoMT
Features: IoMT
Top Stories
INSIDER: Medical
Brain Implant Enables Safer Drug Delivery
Features: Medical
Built to Adapt: Rethinking Contract Manufacturing for a New Era
Podcasts: Design
Smarter Pathways for Precision Drug Delivery in Cancer Care
From the Editor: Medical
From The Editor: Charting the Technologies That Will Shape Medtech in 2026
Features: Materials
OEM Toll Processing Advances High-Value Products
Podcasts: Medical
Ask the Expert
John Chandler on Achieving Quality Motion Control

FAULHABER MICROMO brings together the highest quality motion technologies and value-added services, together with global engineering, sourcing, and manufacturing, to deliver top quality micro motion solutions. With 34 years’ experience, John Chandler injects a key engineering perspective into all new projects and enjoys working closely with OEM customers to bring exciting new technologies to market.
Webcasts
 Podcasts: Medical
Podcasts: Medical
How Wearables Are Enhancing Smart Drug Delivery
 Podcasts: Design
Podcasts: Design
Developing Sustainable Drug-Delivery Devices
 Podcasts: Design
Podcasts: Design
Smarter Pathways for Precision Drug Delivery in Cancer Care
 On-Demand Webinars: Medical
On-Demand Webinars: Medical
Understanding Testing and Compliance Requirements for Wireless Medical Devices
 Podcasts: AR/AI
Podcasts: AR/AI
Personalized Medicine and Drug Delivery
 Podcasts: Manufacturing & Prototyping
Podcasts: Manufacturing & Prototyping
Inside Story
Inside Story: Trends in Packaging and Sterilization

Eurofins Medical Device Testing (MDT) provides a full scope of testing services. In this interview, Eurofins’ experts, Sunny Modi, PhD, Director of Package Testing; and Elizabeth Sydnor, Director of Microbiology; answer common questions on medical device packaging and sterilization.