News: Test & Measurement
Nelson Laboratories, LLC, a business unit of Sotera Health Company and provider of outsourced microbiological and analytical chemistry testing and advisory...
News: Test & Measurement
Nelson Labs, a provider of microbiological testing, analytical chemistry testing, and advisory services, has expanded its state-of-the-art laboratory space at its Wiesbaden,...
Briefs: Test & Measurement
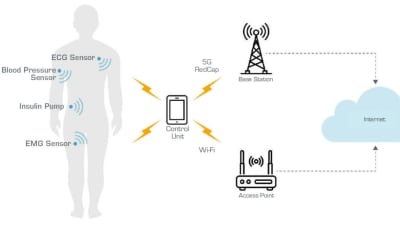
This article presents seven critical questions that medical device designers should be asking as they build the next generation of wireless products to improve quality of life for millions of patients. Read on to learn what they are.
Briefs: Test & Measurement
Developing and evaluating primary packaging for medical devices continues to be an area ripe for innovation, with ample opportunity to improve existing processes. Read on to learn more about the topic.
Inside Story: Medical
Eurofins Medical Device Testing (MDT) provides a full scope of testing services. In this interview, Eurofins’ experts, Sunny Modi, PhD, Director of Package Testing; and Elizabeth Sydnor, Director of Microbiology; answer common questions on medical device packaging and sterilization.
Products: Medical
See which products and services the video spotlight is on, including Dotmatics, an integrated scientific informatics platform that supports research and development activities; an autoinjector testing system featuring precise measurement and control capabilities; BD’s Physiojet disposable autoinjector; testing software that takes over the test sequence with determination of the results; Stevanto Group’s technology centers; and more.
INSIDER: Test & Measurement
A team at Sandia National Laboratories has developed a faster and more comprehensive way of testing personal protective equipment, or PPE. The basic principle: modeling a device...
Supplements: Manufacturing & Prototyping
In our summer edition of the MDB Resource Guide, learn about new cleaning requirements and find the right company to match your medical-design needs.
News: Regulations/Standards
The current U.S. regulatory framework for medical devices under the Center for Devices and Radiological Health (CDRH) includes extensive...
Features: Test & Measurement
In recent years, ethical concerns over the use of live animals in testing have been increasing, particularly for cases not directly related to advancing human health.
Supplements: Electronics & Computers
Learn about the medical manufacturers and cutting-edge applications that stood out in 2022.
Supplements: Manufacturing & Prototyping
In our summer edition of the MDB Resource Guide, learn about new cleaning requirements and find the right company to match your medical-design needs.
Features: Materials
Disinfectant formulations tend to be comprised of an active ingredient, a solvent or aqueous carrier solution, and additional constituents that enhance the formulation’s properties.
Briefs: Test & Measurement
The results from tests on animal brain tissues suggest it could help clinicians to better monitor both disease progression and patients’ response to treatment than is currently possible.
Briefs: Test & Measurement
Trends in wearable technology follow those of the broader biomedical and electronics industries — devices are getting smaller, smarter, and easier to use. Specifically, wearables in...
Features: Test & Measurement
Over the last few decades, additive manufacturing (AM)/ 3D printing has fundamentally changed the way that manufacturers approach product development. Industry is now...
Features: Test & Measurement
The advent of automatic drug-delivery devices has empowered patients to take their treatments in their own hands.
Features: Materials
Learn the key parameters for evaluating the chemical resistance of your materials.
Features: Regulations/Standards
While many devices have received Emergency Use Authorization (EUA) for short-term use, new testing procedures are needed for the long-term.
News: Medical
Sotera Health, a global provider of mission-critical sterilization and lab-testing and advisory services, has acquired Montana-based BioScience Laboratories. This acquisition expands...
News: Medical
Frequent cleaning and surface disinfection — already proven to help reduce the risk of hospital-acquired infections (HAIs) — has become even more critical due to...
INSIDER: Test & Measurement
A CRISPR-based diagnostic for COVID-19 can produce results in 30 minutes to an hour, with similar accuracy as the standard PCR diagnostics now used. STOPCovid could be made cheaply enough that people...
Features: Packaging & Sterilization
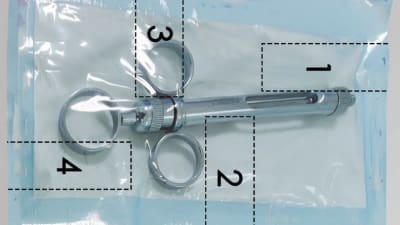
Engineers are thinking of creative ways to design a packaging system that will ensure safe arrival of a medical device.
Products: Software
Simulation Software
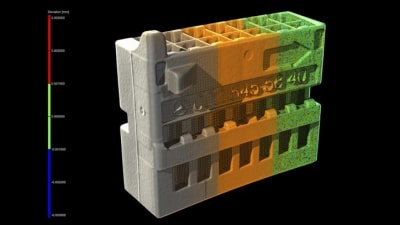
Volume Graphics, Charlotte, NC, has added and augmented important functionalities to its software that help designers and manufacturers capture and interrogate product data to improve final...
Products: Sensors/Data Acquisition
Shielding materials, static control, 3D printing software, and more.
Blog: Medical
A new peer-reviewed study by researchers at NSF International and Novateur Ventures finds significant variability in the accuracy of currently available COVID-19...
Blog: Design
“Testing, testing, testing.” It’s a mantra that health officials have been constantly promoting because screening people for COVID-19 is the best way to contain...
Blog: Test & Measurement
OU Medicine, the OU Health Sciences Center, and the Oklahoma Medical Research Foundation collaborated to create a new test for COVID-19 using technology and...
Blog: Medical
Now that diagnostic companies can sell COVID-19 antibody tests without FDA authorization, healthcare teams should work closely with clinical laboratory experts to...

 Podcasts: Medical
Podcasts: Medical Podcasts: Design
Podcasts: Design Podcasts: Design
Podcasts: Design On-Demand Webinars: Medical
On-Demand Webinars: Medical Podcasts: AR/AI
Podcasts: AR/AI Podcasts: Manufacturing & Prototyping
Podcasts: Manufacturing & Prototyping