News: Medical
Properly prepared test articles are critical for accurate biocompatibility testing. As a sponsor of a medical device, it is imperative for medical device manufacturers to understand what to do to ensure a successful test of a proposed medical device.
Technology Leaders: Medical
Traditionally, toxicologists and biocompatibility experts considered the materials in breathing gas pathways as external communicating devices and evaluated these...
Features: Regulations/Standards
When ISO 9001 was produced by the International Standards Organization, it put forth the general quality standard that organizations could adopt to ensure that the organization is focused on...
Features: Medical
The reliability of the embedded software used in medical devices and the risk associated with it has become a vital concern. IEC 62304, “Medical device software...
Features: Medical
FDA’s introduction to its rules for medical device regulation states: “Medical devices are classified into Class I, II, and III. Regulatory control increases...
Features: Regulations/Standards
The Internet of Things (IoT) has been described as the interconnection via the Internet of computing devices embedded in everyday objects, enabling them to send and receive...
Technology Leaders: Regulations/Standards
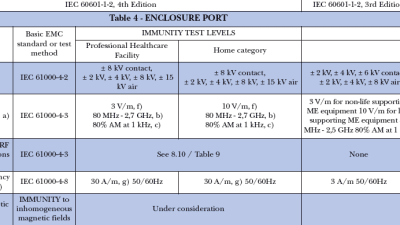
Electromagnetic compatibility (EMC) requirements for medical devices and systems is defined by IEC 60601-1-2. The fourth edition implementation of this EMC standard is...
From the Editor: Regulations/Standards
The industry was buzzing when Stryker announced in July that it would require its future suppliers of critical manufacturing processes to be accredited by MedAccred, a medical supply chain oversight program formed in 2010 by the Performance Review Institute.
From the Editor: Medical
Incoming AdvaMed chairman Nadim Yared will continue AdvaMed’s drive to ensure a permanent repeal of the medical device excise tax, calling it his “highest priority” heading into his term as chairman. Yared, who is president and CEO of CVRx, spoke at a press conference in April.
News: Connectivity
The Food and Drug Administration (FDA) is flagging a new technical information report (TIR) from AAMI that provides crucial guidance for “wireless coexistence” for a wide array of medical devices and systems.
Features: Energy
Safety and reliability are the key concerns when determining the right power source for a medical device. Lithium-ion (Li-ion) batteries are often considered for their higher...
Features: Manufacturing & Prototyping
Design validation is one of the most important aspects of the design and development process for medical devices. It is at this stage that the medical device manufacturer confirms that the device...
Briefs: Regulations/Standards
The U.S. Department of Veterans Affairs (VA) and UL (Underwriters Laboratories) announced a signed Cooperative Research and Development Agreement (CRADA) program to create medical device cybersecurity...
Features: RF & Microwave Electronics
Implementation of IEC 60601-1-2, 4th edition is on the horizon. This collateral standard to the IEC 60601-1 medical safety standard specifies the electromagnetic compatibility (EMC) requirements for medical...
INSIDER: Electronics & Computers
The U.S. Department of Veteran Affairs (VA), Washington, DC, and UL (Underwriters Laboratories), Northbrook, IL, a global safety science organization, have signed a Cooperative Research and Development Agreement (CRADA) for current and emerging medical devices cybersecurity standards...
INSIDER: Medical
The Diabetes Technology Society recently announced its new cybersecurity standard for interconnected diabetes devices called DTSec. The standard specifies performance requirements utilizing the ISO/IEC 15408 framework used to define security requirements on “smart” medical...
Features: Test & Measurement
Manufacturers producing medical devices that involve patient contact are typically required to perform biological safety evaluations, including biocompatibility tests...
INSIDER: Medical
The International Organization for Standardization (ISO), Geneva, Switzerland, late last month released its long-awaited revision to ISO 13485, the global standard for medical device quality management...
Features: Electronics & Computers
When it comes to medical equipment, nothing is more important than the safety of patients and health care personnel. From diagnostic tools such as ultrasound devices to home health...
From the Editor: Medical
According to the late, great David Bowie, “the stars look very different today”. After two years of collecting the 2.3% Medical Device Excise Tax, the tax has now been suspended for all of 2016 and 2017 when President Obama signed the Consolidated Appropriations Act of 2016. The tax was expected to raise almost $30 billion over...
INSIDER: Regulations/Standards
IEEE, Piscataway, NJ, has announced a new standard and two new standards development projects designed to support plug-and-play, interoperable communications across eHealth devices. The new eHealth standard is IEEE 2410™-2015, Biometrics Open Protocol Standard,...
Briefs: Packaging & Sterilization
Ensuring that device packaging meets specifications.
Considering the complex science and research that goes into developing medical devices, it is important...
Technology Leaders: Regulations/Standards
Collaboration among healthcare technology stakeholders—from device manufacturers and healthcare delivery organizations to healthcare security intelligence...
Technology Leaders: Medical
After a 20-year effort to establish standards which would minimize the risk of medical misconnections, the pending release of the ISO 80369 series of standards has now...
Briefs: Test & Measurement
The FDA recently adopted three nanotechnology standards as part of a major update to the administration’s List of Recognized Standards. The documents comprise a Technical Specification (TS) developed...
Features: Regulations/Standards
Particulate testing of cardiovascular medical devices is an important and valuable step...
News: Medical
AAMI, the Association for the Advancement of Medical Instrumentation, is leading the small-bore connectors initiative, an international effort to decrease tubing misconnections and increase patient safety. Recently, AAMI has released two additional standards focusing on how to design connectors...
Features: Test & Measurement
Not only are medical devices expected to function as intended, they must meet ergonomic, safety, FDA and functional requirements. They must be designed to function in adverse...
Features: Medical
Medical device manufacturers operate in a challenging environment filled with stringent regulatory requirements and industry pressures. With a rise in mainstream competitors...

 Podcasts: Medical
Podcasts: Medical Podcasts: Design
Podcasts: Design Podcasts: Design
Podcasts: Design On-Demand Webinars: Medical
On-Demand Webinars: Medical Podcasts: AR/AI
Podcasts: AR/AI Podcasts: Manufacturing & Prototyping
Podcasts: Manufacturing & Prototyping