Features: Transportation
The "Evolving To MedDev" 2021 digital event will provide automotive and aerospace companies with practical information about how they can enter into the medical device supply chain.
Features: Medical
While many devices have received Emergency Use Authorization (EUA) for short-term use, new testing procedures are needed for the long-term.
Technology Leaders: Regulations/Standards
There are no true "medical robots," says an industry expert. Here's why.
Briefs: Wearables
Regulatory changes have allowed these essential healthcare professionals to step in and help out more independently.
From the Editor: Regulations/Standards
FDA just released the agency’s first action plan addressing artificial intelligence (AI) and machine learning (ML).
Blog: Regulations/Standards
Yes, there will be a day when the U.S. EUA, Emergency Use Authorization Act, will be lifted, the medical device industry goes back to reality, and the FDA regains control of the medical approval process with...
Features: Regulations/Standards
A number of manufacturers are still in the process of confirming who qualifies as an "Economic Operator."
Features: Regulations/Standards
Philip Nelson, a partner with Knobbe Martens, provides some insights for device companies facing challenges with intellectual property.
Features: Medical
How to make sense of the 566-page Medical Device Regulation (MDR). This article focuses on a strategy to address material information and what should be included in a gap analysis.
Features: Medical
Why Class I manufacturers should not place European Medical Device Regulation (EU MDR) compliance at the end of their to-do lists.
Blog: Medical
The U.S. Food and Drug Administration (FDA) took action May 25, 2020, to make more ventilators available to healthcare personnel in the United States. FDA issued an emergency use...
Blog: Regulations/Standards
The U.S. Food and Drug Administration took significant action to help increase the availability of ventilators and accessories, as well as other...
Blog: Regulations/Standards
On Wednesday, March 25, 2020 (3–4 p.m. Eastern), the U.S. Food and Drug Administration (FDA) will host a virtual Town Hall for clinical laboratories and commercial manufacturers...
Blog: Regulations/Standards
The Food and Drug Administration (FDA) has issued a new guidance to provide a policy to help expand the availability and capability of noninvasive remote monitoring devices to facilitate patient...
Blog: Medical
The U.S. Food and Drug Administration (FDA) has taken two additional significant diagnostic actions during the coronavirus outbreak (COVID-19) by...
From the Editor: Regulations/Standards
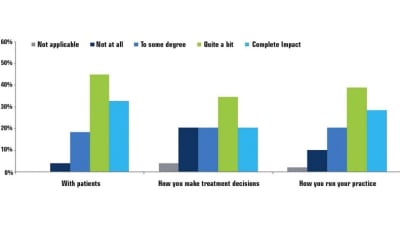
Under new rules to market medical devices in the European Union (EU), only 27 percent of respondents said they will be fully compliant with the regulations...
Features: Regulations/Standards
Companies regulated by the U.S. Food and Drug administration (FDA) need to establish current good manufacturing practices (CGMPs) as part of Title 21 CFR part 820 requirements. This includes...
From the Editor: Regulations/Standards
Spurring Innovation of Brain-Computer Interface Devices FDA is continuing to encourage innovation in novel areas — the latest being novel brain implants, often referred to as brain-computer interface devices — that can help patients with paralysis or amputation gain...
Briefs: Test & Measurement
Calibration of a device is carried out to minimize the uncertainty in measurements. It helps in reducing the errors and brings the measurement to an acceptable level. With...
From the Editor: Regulations/Standards
By the time you read this, Robert M. Califf, MD, a recognized global leader in cardiology, clinical research, and medical economics, will have been installed as the U.S. Food and Drug Administration’s...
INSIDER: Regulations/Standards
The FDA is seeking comments from the medical device industry and healthcare community that refurbish, recondition, rebuild, remarket, remanufacture, service, and repair medical devices.
Features: Regulations/Standards
Some of the biggest stumbling blocks encountered by medical device firms on the way to clearance or approval of their devices by the U.S. Food and Drug...
INSIDER: Medical
Cybersecurity threats to medical devices are a growing concern that present a potential risk to the safety and effectiveness of medical devices. To address this, the FDA has issued...
INSIDER: Medical
In a draft guidance for industry and staff issued on December 31, the FDA proposed notifying the public about medical device “emerging signals that the agency is monitoring or analyzing,...
INSIDER: Regulations/Standards
The FDA’s Center for Devices and Radiological Health (CDRH) announced the 2015 Experiential Learning Program (ELP) General Training Program, which is intended to educate CDRH staff regarding the policies, laboratory practices, and challenges faced in broader disciplines that impact the medical...
INSIDER: Medical
On October 14, the FDA issued a draft guidance document to assist industry in designing evaluation strategies for, and reporting the results of, animal studies for medical devices. The studies utilized for the assessment of these devices typically provide initial evidence of device...
INSIDER: Medical
OpenFDA is releasing a treasure trove of information on medical devices that could help spur innovation and advance scientific research. OpenFDA’s Application Programming Interface (API) expands the previous resources about medical device-related adverse events and recalls by incorporating information...
INSIDER: Regulations/Standards
The FDA is seeking companies to take part in a pilot program that would allow medical device manufacturers to report malfunctions of certain low- and medium-risk devices on a quarterly basis. This pilot program will help the agency develop criteria for quarterly malfunction reporting...
From the Editor: Medical
In mid-July, the House of Representatives passed HR 6, also known as the 21st Century Cures Act, which expedites research and development on debilitating diseases and makes it easier to get important treatments to the patients who need them. Among other things, it makes research collaborations easier, reforms and streamlines...

 Podcasts: Medical
Podcasts: Medical Podcasts: Design
Podcasts: Design Podcasts: Design
Podcasts: Design On-Demand Webinars: Medical
On-Demand Webinars: Medical Podcasts: AR/AI
Podcasts: AR/AI Podcasts: Manufacturing & Prototyping
Podcasts: Manufacturing & Prototyping