Briefs: Medical
The FDA has taken a substantial step in its digital modernization strategy with the deployment of agentic AI capabilities across all agency employee groups. The move represents an expansion of the agency’s internal AI tools, intended to streamline complex, multi-step processes that support regulatory science, product review, and compliance activities. Read on to learn more.
From the Editor: Regulations/Standards
"If regulators are accelerating their digital transformation, the medtech community must be ready to meet them there." Read more about Editor and Director of Medical Content Sherrie Trigg's opinion in From the Editor.
Products: Medical
See the product of the month: SGS has received EPA approval as a recognized third-party certification body for testing and certification of magnetic resonance imaging (MRI) machines under the ENERGY STAR® label. The certification allows brands and manufacturers to demonstrate that a device uses less energy in ready to scan mode as well as via an automated power down to an energy saving low power state. Read on to learn more.
Trivia: Medical
In May 2004, FDA issued a guidance document that reclassified certain dental implants from Class III to Class II, thereby reducing regulatory burdens while maintaining safety standards. What type of dental implants were...
Briefs: Packaging & Sterilization
MedAccred is an industry-managed, consensus-driven approach to ensuring critical manufacturing process quality throughout the medical device supply chain. The MedAccred program is administered by PRI and governed by the original equipment manufacturer (OEM) subscribers who define program requirements, review audit reports, and accept non-conformance resolutions. Read on to learn more.
News: Regulations/Standards
In a significant collaboration to help advance pediatric health, Children’s National Hospital and the U.S. Food and Drug Administration’s (FDA) Office of...
From the Editor: Regulations/Standards
Hear directly from Sherrie Trigg, Editor and Director of Medical Content, about the FDA's final ruling on LDTs and why that is raising concern.
From the Editor: Medical
At the end of June, FDA released a draft guidance that is designed to facilitate and streamline development of stand-alone devices and combination products by improving the consistency of drug-delivery performance information included in applications and submissions. Read on to learn more about it.
Blog: Medical
The global landscape for medical technology, biotechnology, and life sciences is on the cusp of seismic change.
From the Editor: Regulations/Standards
Analytics firm GlobalData has weighed in on the U.S. FDA's proposal to reclassify laboratory-developed tests (LDTs) as medical devices. FDA historically exercised enforcement discretion over LDTs, meaning that it did not actively regulate them like it does for other medical devices, so this is a significant regulatory change.
Briefs: Design
Neither the MDR nor Annex XVI are new. Nevertheless, the entry into force of the common specifications now presents a new situation for manufacturers of devices without an intended medical purpose.
Blog: Medical
Understanding what the FDA really means by their time frames, knowing how to address their regulatory issues, and preparing a response to a 483 or Warning Letter is critical to showing the FDA that you understand what they expect from you and that you will work to bring your company into compliance. Another critical point is to understand that you can negotiate with the agency.
News: Regulations/Standards
The U.S. Food and Drug Administration has created a new Digital Health Advisory Committee to help the agency explore the complex, scientific and technical issues related to...
News: Manufacturing & Prototyping
The U.S. Food and Drug Administration (FDA) updated its Catalog of Regulatory Science Tools by adding four new tools and updating nine others. The catalog is a peer-reviewed resource...
News: Regulations/Standards
TÜV SÜD has received approval as a UK Approved Body (UKAB) for medical devices. With this approval, medical device manufacturers can implement UKCA certification for the UK market and CE...
Briefs: Regulations/Standards
Thanks to artificial intelligence (AI), augmented reality (AR) has long shaped product development across a variety of areas, including the medtech industry.
News: Medical
An agreement reached by the UK and EU regarding the Windsor Framework brings changes to the Northern Ireland regulatory landscape. The agreement , in principle, was reached on...
News: Regulations/Standards
The current U.S. regulatory framework for medical devices under the Center for Devices and Radiological Health (CDRH) includes extensive...
Supplements: Connectivity
In our summer edition of the MDB Resource Guide, learn about new cleaning requirements and find the right company to match your medical-design needs.
Supplements: Regulations/Standards
In our summer edition of the MDB Resource Guide, learn about new cleaning requirements and find the right company to match your medical-design needs.
Features: Mechanical & Fluid Systems
Although the pandemic has delayed the MDR’s validation date, it has not diminished the importance of this new regulation.
Technology Leaders: Regulations/Standards
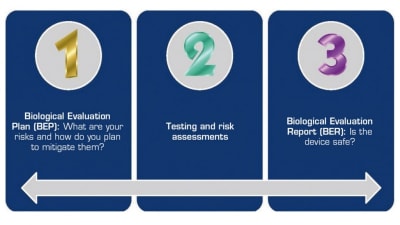
Modern evaluation of biocompatibility uses a risk-based approach.
Features: Regulations/Standards
Bring your project from the process development phase of manufacturing to a fully validated one.
From the Editor: IoMT
New best practices for understanding medical device security.
Supplements: Materials
In our winter edition of the MDB Resource Guide, learn about new cleaning requirements and find the right company to match your medical-design needs.
Products: Manufacturing & Prototyping
Linear stepper stages, tubing, Bluetooth modules, and more.
Features: Regulations/Standards
A robust, optimized mechanical testing program is essential to successful quality control testing.
Briefs: Regulations/Standards
Deadlines are approaching for compliance.
Supplements: Data Acquisition
Our 2021 Resource Guide shows you the top manufacturers in materials, manufacturing, and a range of other medical-device categories.

 Podcasts: Medical
Podcasts: Medical Podcasts: Design
Podcasts: Design Podcasts: Design
Podcasts: Design On-Demand Webinars: Medical
On-Demand Webinars: Medical Podcasts: AR/AI
Podcasts: AR/AI Podcasts: Manufacturing & Prototyping
Podcasts: Manufacturing & Prototyping