Briefs: AR/AI
The FDA has taken a substantial step in its digital modernization strategy with the deployment of agentic AI capabilities across all agency employee groups. The move represents an expansion of the agency’s internal AI tools, intended to streamline complex, multi-step processes that support regulatory science, product review, and compliance activities. Read on to learn more.
From the Editor: AR/AI
"If regulators are accelerating their digital transformation, the medtech community must be ready to meet them there." Read more about Editor and Director of Medical Content Sherrie Trigg's opinion in From the Editor.
Trivia: Medical
In May 2004, FDA issued a guidance document that reclassified certain dental implants from Class III to Class II, thereby reducing regulatory burdens while maintaining safety standards. What type of dental implants were...
From the Editor: Regulations/Standards
The U.S. Senate's bipartisan legislation to expedite Medicare coverage of FDA-breakthrough-designated medical technologies and diagnostic tests could significantly impact the medical device industry and manufacturers, streamlining processes for breakthrough devices, enhancing patient access, and reducing barriers to market entry. Read on to see what Sherrie Trigg, Editor and Director of Medical Content, thinks about the matter.
From the Editor: Regulations/Standards
Hear directly from Sherrie Trigg, Editor and Director of Medical Content, about the FDA's final ruling on LDTs and why that is raising concern.
Blog: Medical
Recently, I came across an FDA regulatory situation you do not see too often. On the same day, FDA sent two teams of field investigators into two geographically different sites of the same company to...
Trivia: Regulations/Standards
What government group was formed to study medical devices and recommend legislation, ultimately leading to the 1976 Medical Device Amendments?
Podcasts: Regulations/Standards
In this season, we are taking an in-depth look at Printed Health: Transforming Medicine with Advanced Manufacturing, and this episode will focus specifically on...
From the Editor: Regulations/Standards
At the end of June, FDA released a draft guidance that is designed to facilitate and streamline development of stand-alone devices and combination products by improving the consistency of drug-delivery performance information included in applications and submissions. Read on to learn more about it.
INSIDER: Regulations/Standards
On Tuesday, February 6 at 12:30 pm, Balazs Boznik, BSEE, Technical Director, Medical Audit, at SGS North America will discuss FDA’s plan to adopt ISO 13485.
From the Editor: Regulations/Standards
Analytics firm GlobalData has weighed in on the U.S. FDA's proposal to reclassify laboratory-developed tests (LDTs) as medical devices. FDA historically exercised enforcement discretion over LDTs, meaning that it did not actively regulate them like it does for other medical devices, so this is a significant regulatory change.
News: Regulations/Standards
MasterControl, a provider of quality and manufacturing software solutions for the life sciences industry, has released a new study analyzing five years of product recall data from...
Blog: Medical
Understanding what the FDA really means by their time frames, knowing how to address their regulatory issues, and preparing a response to a 483 or Warning Letter is critical to showing the FDA that you understand what they expect from you and that you will work to bring your company into compliance. Another critical point is to understand that you can negotiate with the agency.
News: Medical
The U.S. Food and Drug Administration has created a new Digital Health Advisory Committee to help the agency explore the complex, scientific and technical issues related to...
News: Design
To foster the development and commercialization of medical devices designed for children, the Food and Drug Administration (FDA) has awarded a nearly $7.5 million grant to...
News: Design
Organ transplant developer, Paragonix Technologies has received FDA clearance for its next-generation donor lung preservation system, BAROguard™....
News: Regulations/Standards
The U.S. Food and Drug Administration (FDA) updated its Catalog of Regulatory Science Tools by adding four new tools and updating nine others. The catalog is a peer-reviewed resource...
News: Medical
The current U.S. regulatory framework for medical devices under the Center for Devices and Radiological Health (CDRH) includes extensive...
News: Medical
Movano Health, Pleasanton, CA, has announced successful preliminary results of its pivotal hypoxia trial, which was completed in conjunction with the...
Supplements: Materials
In our summer edition of the MDB Resource Guide, learn about new cleaning requirements and find the right company to match your medical-design needs.
Supplements: Connectivity
In our summer edition of the MDB Resource Guide, learn about new cleaning requirements and find the right company to match your medical-design needs.
Technology Leaders: Test & Measurement
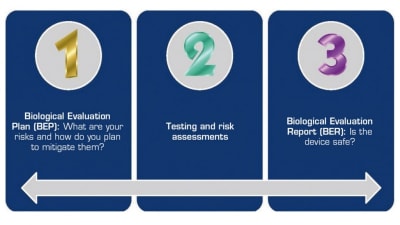
Modern evaluation of biocompatibility uses a risk-based approach.
Features: Medical
Bring your project from the process development phase of manufacturing to a fully validated one.
From the Editor: Connectivity
New best practices for understanding medical device security.
Supplements: Materials
In our winter edition of the MDB Resource Guide, learn about new cleaning requirements and find the right company to match your medical-design needs.
Features: Regulations/Standards
A robust, optimized mechanical testing program is essential to successful quality control testing.
Briefs: Manufacturing & Prototyping
Deadlines are approaching for compliance.
Supplements: Robotics, Automation & Control
Our 2021 Resource Guide shows you the top manufacturers in materials, manufacturing, and a range of other medical-device categories.
Features: Design
The "Evolving To MedDev" 2021 digital event will provide automotive and aerospace companies with practical information about how they can enter into the medical device supply chain.

 Podcasts: Medical
Podcasts: Medical Podcasts: Design
Podcasts: Design Podcasts: Design
Podcasts: Design On-Demand Webinars: Medical
On-Demand Webinars: Medical Podcasts: AR/AI
Podcasts: AR/AI Podcasts: Manufacturing & Prototyping
Podcasts: Manufacturing & Prototyping