From the Editor: Medical
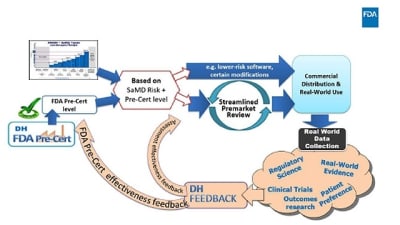
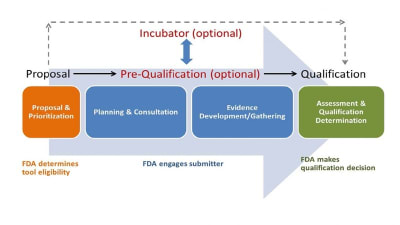
From the Editor — Spurring Innovation of Brain-Computer Interface Devices
From the Editor: Medical
From the Editor: AR/AI
From the Editor: Energy
From the Editor: Medical
From the Editor: Software
From the Editor: Manufacturing & Prototyping
From the Editor: Medical
From the Editor: AR/AI
From the Editor: Medical
From the Editor: AR/AI
From the Editor: Medical
From the Editor: Packaging & Sterilization
From the Editor: Manufacturing & Prototyping
From the Editor: Medical
From the Editor: Medical
From the Editor: Medical
From the Editor: AR/AI
From the Editor: Medical
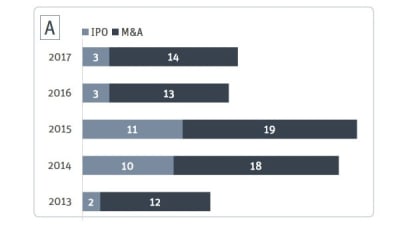
Medtech M&A on the Rise Again
From the Editor: Medical
MedAccred Makes Its Mark
From the Editor: Medical
Your Cyberattack Wake-Up Call
From the Editor: Medical
The Internet of Dangerous Things
From the Editor: Medical
Permanent Device Tax Repeal Is ‘Highest Priority’
From the Editor: Medical
Confidence High, but Concerns Remain
From the Editor: Medical
And the Winners Are...
From the Editor: Medical
From the Editor: Medical
From the Editor: Communications
Building on Success
From the Editor: Medical
Good News/Sad News
Top Stories
INSIDER: Medical
Tiny Sensor Could Transform Head Injury Detection
News: Robotics, Automation & Control
Quantum Surgical Acquires NeuWave Medical, Inc.
Briefs: Manufacturing & Prototyping
Shrinking Materials Hold Big Potential for Smart Devices
INSIDER: Design
MD&M West Presentation: How Intelligent Product Lifecycle Management...
Podcasts: Medical
Surgical & Assistive Robotics: Redefining Human Capability
INSIDER: Medical
MD&M West Keynote: AI Without the Hype: Real Results in Manufacturing
Ask the Expert
Dan Sanchez on How to Improve Extruded Components

Improving extruded components requires careful attention to a number of factors, including dimensional tolerance, material selection, and processing. Trelleborg’s Dan Sanchez provides detailed insights into each of these considerations to help you advance your device innovations while reducing costs and speeding time to market.
Webcasts
 Podcasts: Robotics, Automation & Control
Podcasts: Robotics, Automation & Control
The Hidden Nervous System of Surgical Robotics: Power, Data & Sensing Behind...
 Podcasts: Medical
Podcasts: Medical
Surgical & Assistive Robotics: Redefining Human Capability
 Podcasts: Medical
Podcasts: Medical
How Wearables Are Enhancing Smart Drug Delivery
 Podcasts: Design
Podcasts: Design
Developing Sustainable Drug-Delivery Devices
 Podcasts: Medical
Podcasts: Medical
Smarter Pathways for Precision Drug Delivery in Cancer Care
 On-Demand Webinars: Manufacturing & Prototyping
On-Demand Webinars: Manufacturing & Prototyping
Understanding Testing and Compliance Requirements for Wireless Medical Devices
Inside Story
Inside Story: Trends in Packaging and Sterilization

Eurofins Medical Device Testing (MDT) provides a full scope of testing services. In this interview, Eurofins’ experts, Sunny Modi, PhD, Director of Package Testing; and Elizabeth Sydnor, Director of Microbiology; answer common questions on medical device packaging and sterilization.