Stories
Products: Medical
Products: Medical
Briefs: Medical
Briefs: IoMT
Briefs: IoMT
Supplements: Materials
Supplements: Regulations/Standards
Briefs: Connectivity
Features: Medical
Supplements: Sensors/Data Acquisition
Supplements: IoMT
Features: AR/AI
Supplements: Mechanical & Fluid Systems
Products: Electronics & Computers
Features: Regulations/Standards
Features: Wearables
Features: RF & Microwave Electronics
Features: IoMT
INSIDER: Medical
INSIDER: Wearables
Features: Test & Measurement
News: IoMT
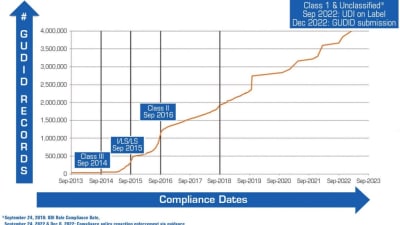
FDA to Address New “Quik” Review Program Pilot for Faster Review of Certain 510(k)s
Features: IoMT
Briefs: Wearables
Features: IoMT
Features: Medical
Features: IoMT
Features: IoMT
Features: Regulations/Standards
Top Stories
INSIDER: Medical
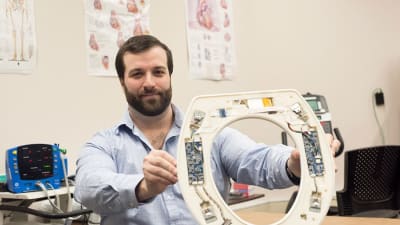
Brain Implant Enables Safer Drug Delivery
Features: Medical
Built to Adapt: Rethinking Contract Manufacturing for a New Era
Podcasts: Design
Smarter Pathways for Precision Drug Delivery in Cancer Care
From the Editor: Medical
From The Editor: Charting the Technologies That Will Shape Medtech in 2026
Features: Materials
OEM Toll Processing Advances High-Value Products
Podcasts: Medical
Ask the Expert
Dan Sanchez on How to Improve Extruded Components

Improving extruded components requires careful attention to a number of factors, including dimensional tolerance, material selection, and processing. Trelleborg’s Dan Sanchez provides detailed insights into each of these considerations to help you advance your device innovations while reducing costs and speeding time to market.
Webcasts
 Podcasts: Medical
Podcasts: Medical
How Wearables Are Enhancing Smart Drug Delivery
 Podcasts: Design
Podcasts: Design
Developing Sustainable Drug-Delivery Devices
 Podcasts: Design
Podcasts: Design
Smarter Pathways for Precision Drug Delivery in Cancer Care
 On-Demand Webinars: Medical
On-Demand Webinars: Medical
Understanding Testing and Compliance Requirements for Wireless Medical Devices
 Podcasts: AR/AI
Podcasts: AR/AI
Personalized Medicine and Drug Delivery
 Podcasts: Manufacturing & Prototyping
Podcasts: Manufacturing & Prototyping
Inside Story
Inside Story: Trends in Packaging and Sterilization

Eurofins Medical Device Testing (MDT) provides a full scope of testing services. In this interview, Eurofins’ experts, Sunny Modi, PhD, Director of Package Testing; and Elizabeth Sydnor, Director of Microbiology; answer common questions on medical device packaging and sterilization.